Biomedical Engineering Reference
In-Depth Information
An
Escherichia coli
suspension with a concentration of 10
6
CFU/mL is employed to assess and
compare the antimicrobial properties of the Cu PIII PE and Cu/N
2
PIII PE samples at immersion
time periods of 0, 14, and 28 days (Figure 19.53b). Before they are immersed in SBF (i.e., day 0), both
Cu PIII PE and Cu/N
2
PIII PE have excellent antibacterial effects against
E. coli
, that is, 96.2% and
95.5%, respectively. This mainly stems from the surface-deposited Cu that can deliver immediate
antimicrobial effects. After immersion in SBF for 14 days, the Cu PIII PE and Cu/N
2
PIII PE still
possess good antibacterial performances against such a high cell suspension in spite of the reduced
Cu release rates. The antimicrobial effects against
E. coli
are 70.6% and 84.3%, respectively. It should
be noted that the Cu/N
2
PIII PE sample exhibits better antibacterial effects. The difference is even
more evident after immersion for 28 days. The results thus unequivocally demonstrate the excellent
antibacterial effect of Cu/N
2
PIII. The process allows for the continuous release of buried Cu to retain
the surface antibacterial ability for a longer period of time. In separate experiments, the prepared
samples were stored under room temperature in air for 6 weeks and their antimicrobial effects were
compared with those of freshly prepared samples. The antimicrobial effects against
E. coli
were
found to hardly change, thus indicating excellent long-lasting effects during normal storage.
It is well known that the antimicrobial properties are primarily related to the release of the
antibacterial reagent, Cu. In the absence of gettering effects, Cu out-diffusion from the substrate is
believed to follow Fick's fi rst law of diffusion [207,208]:
d
N
DS
d
C
d
x
,
___
___
d
t
=
-
(19.4)
where
N
is the amount of copper, d
C
/d
x
is the Cu concentration gradient with distance,
t
is the time,
S
is the surface area of the samples, and
D
is the diffusion coeffi cient. Here,
S
is the same
and d
C
/d
x
is more or less the same because the same Cu PIII conditions are used for both the samples. Conse-
quently, the Cu diffusion rate, d
N
/d
x
, depends mainly on the diffusion coeffi cient,
D
. A diffusion
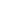
process from the substrate consisting of two zones is being proposed, as schematically described in
Figure 19.54. Diffusion in zone B is described by the above equation whereas that in zone A also
depends on the chemistry and gettering effects between Cu, N, and the PE matrix.
Plasma immersion ion implantation is an effective method to introduce a large quantity of metal
inorganic antimicrobials like Cu into organic medical polymers such as PE up to a depth of several hun-
dred nanometers without causing appreciable damage to the polymer matrix. The use of N
2
PIII in con-
cert with Cu PIII produces new polar unsaturated functional groups such as C
N in the
near-surface of the polymer. They play an important role in regulating the out-diffusion rate of Cu and
prolonging the antibacterial effects signifi cantly. It demonstrates that an inorganic antimicrobial agent
can be effectively incorporated into an organic biomedical polymer and by using a nitrogen plasma
=
N and
-
C
≡
Cu
Polyethylene
Cu
SBF
Cu
Cu
B
A
FIGURE 19.54
Schematic diagram illustrating two zones in the Cu-implanted polyethylene. (From Zhang,
W., Zhang, Y.H., Ji, J.H., Yan, Q., Huang, A.P., and Chu, P.K.,
J. Biomed. Mater. Res.: Part
A
, in press
DOI.10.2002. With permission.)