Biomedical Engineering Reference
In-Depth Information
(ICPMS). The Ni and Ti concentrations released from the control sample are 30.2324 and
0.1575 ppm, respectively. The Ni concentrations from the nitrogen-, acetylene-, and oxygen-
implanted samples are only 0.0117, 0.0082, and 0.0123 ppm, respectively. The Ti concentrations
from the nitrogen- and acetylene-implanted samples are 0.0527 and 0.0057 ppm, respectively. The
Ti concentration from the oxygen-implanted sample is undetectable. The results reveal that the
amounts of Ni leached from all the treated samples are signifi cantly reduced. The leached amount
is only about 0.03-0.04% of that of the control sample.
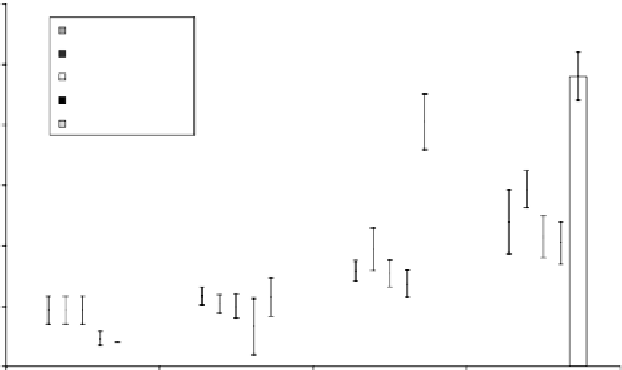
All the plasma-implanted samples are well tolerated by the EGFP-expressing osteoblasts as
shown in Figure 19.28. After culturing for 2 days, the cells start to attach to and proliferate on
all the samples. After 4 days, cell proliferation on the untreated NiTi alloy samples was slightly
higher than that of the nitrogen, oxygen, and acetylene PIII samples. However, the nitrogen PIII
samples exhibit the highest degree of cell proliferation among the samples after 6 and 8 days of
culturing. Cell proliferation on the acetylene- and oxygen-implanted samples was slightly lower
than that on the NiTi control sample after 6 and 8 days, but the difference not signifi cant.
Oxygen, nitrogen, or acetylene PIII can effectively suppress the leaching of nickel from the
NiTi alloys. The enhancement phenomenon can be attributed to the high affi nities of Ti toward
N, C, and O as compared to Ni under high-temperature annealing. It provides a driving force to
enrich the surface with the element forming a stronger chemical bond. The heat of formation of
the lowest titanium oxide is
913 kJ/mole
while that of NiO is
244 kJ/mole
[137]. The heat of
-
-
305.6 kJ/mole
while nickel nitrides such as TiN
3
TiN are unstable with respect
to TiN [138]. The heat of formation of TiC is
formation of TiN is
-
773 kJ/mole
[139] while that of NiC is not well
-
established since the Ni
C phase diagram does not show stable carbides. The term nickel carbide
may only stand for interstitial solid solutions of C in Ni, which possess the NaCl structure [140].
Therefore, the formation of titanium oxide, nitride, and carbide is energetically favored over the
nickel counterparts and this is believed to account for the suppression of Ni in the implanted and
annealed region. It should be noted that the degree of suppression does depend on the implanta-
tion parameters as reported by Tian et al. [137] in their study of the suppression of nickel in the
stainless steel surface after nitrogen PIII.
-
30
NiTi
NiTi-N
NiTi-O
NiTi-C implanted
Empty well
25
20
15
10
5
0
2
4
6
8
Days
FIGURE 19.28
Cell proliferation on various NiTi alloys versus number of days. (From Yeung, K.W.K. et al.,
J. Biomed. Mater. Res.
,
72A, 238, 2005. With permission.)