Biomedical Engineering Reference
In-Depth Information
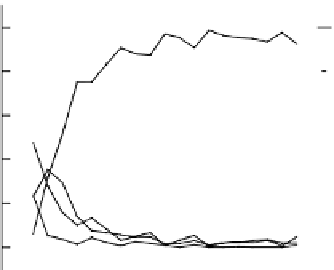
The elemental depth profi les acquired from Na PIIID titanium prepared at chamber pres-
sures of 4
10
-
2
Pa are displayed in Figure 19.23 (left). It should be noted
that PIIID is different from beam-line ion implantation in the sense that both deposition and
implantation take place in the former process. The resulting depth profi le thus consists of both
high-surface concentration Na and an implanted component, as shown in Figure 19.23 (left). In
high-temperature oxidation, sodium titanate is formed by releasing sodium into the solution dur-
ing hydroxylation and leaving titanium hydroxide groups on the surface. The surface chemistry
is the same as that on sodium ion beam-implanted titanium [73], except for a smaller projected
range in the PIIID-treated samples. The process of oxidation, hydroxylation, and leaching of
alkali metal is more complete in the more deeply ion-implanted sample 2 than the mainly depos-
ited sample 1, as shown in Figure 19.24 (right). The surface composition of the NaOH-treated
titanium has been analyzed in detail [75,76]. It consists of a porous, amorphous layer mainly
composed of sodium titanate.
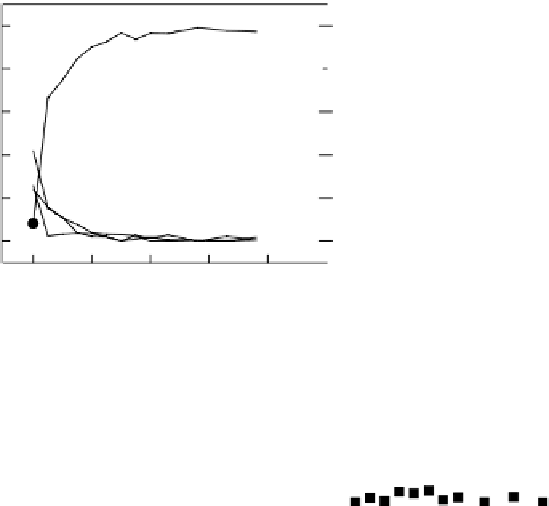
Precipitation of calcium phosphate was evaluated using SBF treatment and the results are
shown in Figure 19.24. The beam line-implanted sample (Ti-II) exhibits very poor bioactivity,
which is even worse than that of the untreated titanium (Ti). Nonetheless, it is very clear that the
PIIID titanium sample (Ti PIII) induces signifi cantly higher CaP precipitation and the effi cacy is
even better than that observed on the NaOH-treated sample. This phenomenon may be attributed to
10
-
2
Pa and 8
×
×
Element profiles after
oxidation and hydroxylation
Element profiles
as implanted
100
Titanium
Oxygen
Sodium
Carbon
Sample 1
4
80
10
−
2
Pa
×
60
40
20
0
100
Sample 2
8
×
10
−
2
Pa
80
60
40
20
0
0
200
400
600
800
0
200
400
600
800
Sputter time (s)
Sputter time (s)
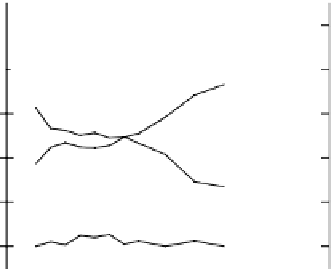
FIGURE 19.23
Depth profi les of the two samples implanted by Na PIIID at two pressure conditions as
implanted and after oxidation and hydroxylation. (From Maitz, M.F. et al.,
Biomaterials
, 26, 5465, 2005. With
permission.)