Biomedical Engineering Reference
In-Depth Information
(a)
(b)
Outermost layer
Higher magnification
100 nm
200 nm
200 nm
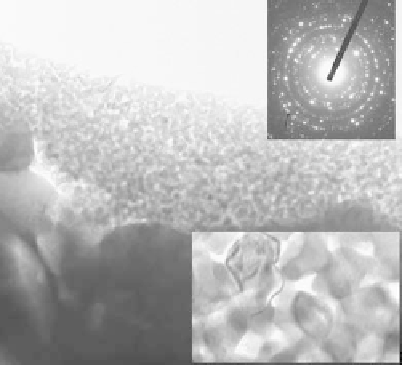
FIGURE 19.15
Cross-sectional TEM views of the as-sprayed nano-TiO
2
coating: (a) coating surface and
(b) coating interior. (From Liu, X.Y. et al.,
Biomaterials
, 26, 6143, 2005. With permission.)
(a)
(b)
Apatite layer
TiO
2
coating
Resin
Electron image 1
30
µ
m
FIGURE 19.16
Surface (a) and cross-sectional (b) views of the hydrogen PIII nano-TiO
2
coating after soak-
ing in SBF for 2 weeks. (From Liu, X.Y. et al.,
Biomaterials
, 26, 6143, 2005. With permission.)
nanostructured surface possesses apatite formability. It can thus be inferred that the bioactivity
of the plasma-sprayed TiO
2
coating depends on two factors: nanostructured surface composed of
enough small particles and hydrogen incorporation.
It has been suggested that OH groups on ceramic surfaces are effective in inducing the forma-
tion of an apatite layer. For instance, gel-derived TiO
2
has been shown to induce surface apatite
formation, but single crystal anatase and titania synthesized by hydrothermal methods cannot do so
[65]. The difference is believed to be because of the Ti
Ti-OH functional groups forming a negatively
charged surface on the titania gel. It is also believed to be one of the reasons for the surface bioactiv-
ity of the hydrogen PIII nano-TiO
2
and the lack of OH groups on the as-sprayed TiO
2
coatings.
The as-sprayed TiO
2
coating is highly oxygen-defi cient. While the outermost surface of the
as-sprayed TiO
2
coating can be immediately reoxidized via oxygen adsorption after it is exposed
to air [66], the subsurface region in the coating is still oxygen-defi cient. During hydrogen PIII,
hydrogen ions react with the outermost bridge oxygen to form Ti
-
Ti-OH bonds because the reaction
is energetically favorable, and two Ti(IV) are reduced to Ti(III) [67]. Eventually, a hydrogenated
-