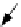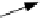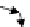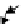Biomedical Engineering Reference
In-Depth Information
is independent of sample size with the proper control of the plasma sheath by varying the plasma
density, bias potential, pulse duration, and confi gurations.
19.2.2 I
ON
-S
OLID
I
NTERACTIONS
I
NDUCED
BY
I
ON
I
MPLANTATION
The important considerations in any description of ion-solid interactions are the depth distribu-
tion of the implanted ions, ion irradiation damage and sputtering, novel synthesis and formation of
materials by irradiation, as well as ion alloying [13-15]. An implanted ion penetrates a solid, slows
down, and comes to rest. The total length of the ion trajectory in the solid is called the ion range
R
.
As the incident ion makes many collisions with lattice atoms and displaces them from their lattice
sites, these displaced atoms can in turn displace others, and the net result is the production of a
highly disordered region along the path of the ion. At suffi ciently high ion doses, these individual
disordered regions may overlap, and an amorphous or metastable crystalline layer may form.
Ion irradiation is quite effi cient in forming vacancy-interstitial pairs. The atomic displacements
resulting from energetic recoiled atoms can be highly concentrated into small localized regions
containing a large concentration of defects that are well in excess of the equilibrium value. If the
defects are produced at temperatures at which they are mobile and can in part be annealed out, the
balance between the rates of formation and annihilation leads to a steady state of excess concentra-
tion of defects. Since the atomic diffusivity is proportional to the defect concentration, an excess
concentration of the defects leads to an enhancement in the diffusion process [16,17]. Thus, the fi nal
ion stopping range and the modifi ed layer can be enhanced and extended by diffusion as shown in
the right side of Figure 19.6. Experiments have shown that ordered alloys could become chemically
disordered under irradiation. The effects of irradiation on the ordered alloy are described by the
competing processes of chemical disordering, which is induced by atomic replacements resulting
from displacement and cascade damage and chemical ordering, which is stimulated by radiation-
enhanced diffusion [18,19]. When an ordered alloy is disordered by exposure to particle irradiation,
thermodynamics will determine the strength of the driving force for the recovery of the chemical
disorder. A driving force will always exist in alloys where the disordered state will not be in the
equilibrium condition, and the primary limitation to reordering will be kinetic.
Sputtered particle
(
±
ion or neutral)
Concentration
Vacuum
Solid
Ion penetration
depth,
R
Implanted
Irradiation-
enhanced diffusion
FIGURE 19.6
Schematic diagram of ion-solid interactions: the sputtering process and the irradiation-
enhanced diffusion process. (From Borg, R.J. and Dienes, G.J.,
An Introduction to Solid State Diffusion
,
Academic Press, Boston, MA, 1988; Thompson, M.W.,
Defects and Radiation Damage in Metals
, Cambridge
University Press, Cambridge, MA, 1969. With permission.)