Biomedical Engineering Reference
In-Depth Information
C
=
O
NH
2
C
=
O
NH
2
NH
2
NH
2
NH
2
NH
NH
NH
2
SWCNT in DMF
DCC
s
s
s
s
s
s
s
s
FAD
FAD
NH
C
=
O
C
=
O
Apo-GOD
C
=
O
NH
2
C
=
O
C
=
O
NH
2
C
=
O
C
=
O
C
=
O
GOD
FAD
NH
NH
2
NH
NH
2
NH
NH
NH
2
NH
NH
NH
2
s
s
s
s
s
s
s
s
s
s
s
s
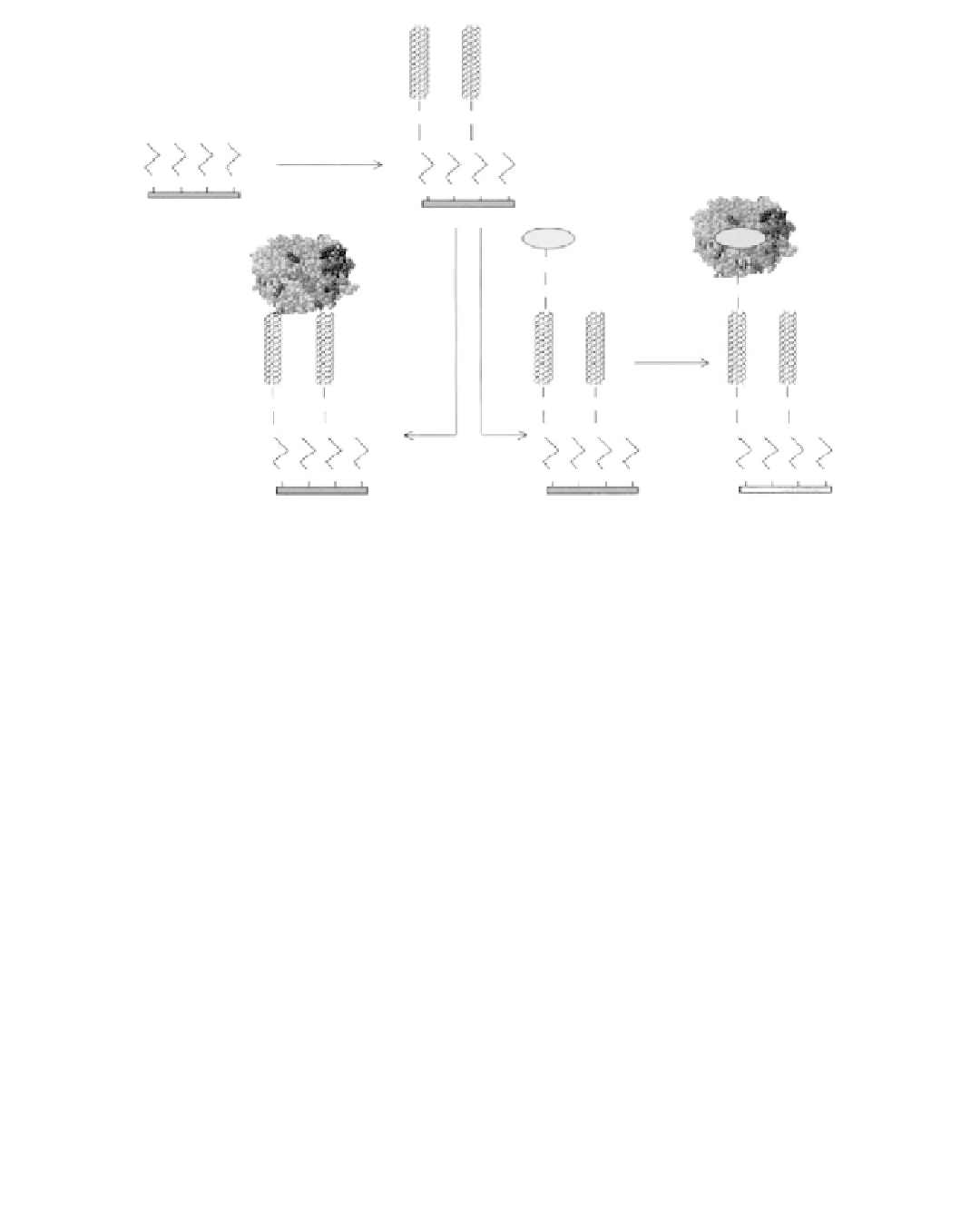
FIGURE 14.7
A schematic representation of the procedure for the modifi cation of a SA monolayer modifi ed
Au electrode with aligned SW CNTs and their subsequent modifi cation to allow direct electron transfer to
GOD. (From Liu, J.Q., Chou, A., Rahmat, W., Paddon-Row, M.N., and Gooding, J.J.,
Electroanalysis
, 17, 38,
2005. With permission.)
been explored. The electrical conducting polymers possess numerous features that allow them to
act as excellent materials for immobilization of biomolecules and rapid electron transfer for effi cient
biosensors. Conducting polymers have conjugated backbone that can be oxidized or reduced by
electron acceptors or donors, resulting in p-doping or n-doping materials, respectively. By adjusting
the doping level, control of electronic property over the entire range from insulator to semiconduc-
tor and then to metal is feasible [204].
The conducting polymers can be produced by various techniques, for example, spin coat-
ing [205], SA monolayer [206], and Langmuir-Blodgett (LB) [204] methods. However, the most
widely used technique is electrochemical synthesis because of its simplicity and reproducibility.
Electrochemical polymerization occurs when suitable monomers are electrochemically oxidized
to create active monomeric and dimeric species that react with each other to form a conjugated
polymer backbone.
A number of favorable characteristics of conducting polymers for the application of biosensors
are as follows: (1) direct and easy deposition on sensor electrode at room temperature by electro-
chemical oxidation of monomer, (2) control of thickness by varying either the potential or current
with time, (3) modulation of the required electronic and mechanical properties by chemical mod-
eling and synthesis, and (4) redox conductivity and polyelectrolyte characteristics of the polymer
useful for sensor applications [207,208].
Conducting polymers are suitable matrix of enzymes because of their excellent electrical con-
ductivity and favorable compatibility with biological molecules in neutral aqueous solutions. Organic
conducting polymers as a convenient ingredient not only immobilize biomolecules, but also provide
a microenvironment that can facilitate the direct electron transfer between biomolecules and the
electrode. The majority of work has been carried out by redox or electronic conducting polymers,
such as polypyrrole (PPy), polyaniline (PANI), and polythiophene.