Biomedical Engineering Reference
In-Depth Information
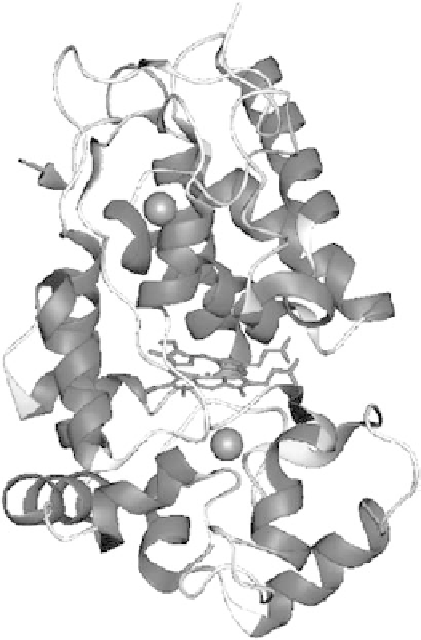
FIGURE 14.1
Three-dimensional representation of the x-ray crystal structure of HRP isoenzyme C. The
heme group is located between the distal and proximal domains, each of which contains a calcium atom.
(From Veitch, N.C.,
Phytochem.
, 65, 249, 2004. With permission.)
Moreover, HRP usually works together with oxidases to construct biosensors, since it can cata-
lyze the redox reaction of H
2
O
2
generated by oxidases at a relatively low potential. For example,
HRP is coimmobilized with GOD for glucose detection [72], with COD for cholesterol detection
[73], and with l-glutamate oxidase (GLOD) for the detection of l-glutamate and alanine amino-
transferase in blood [74].
14.1.1.5 Lactate Dehydrogenase
Lactate dehydrogenase (LDH, E.C. 1.1.1.27) is a kind of dehydrogenase that widely exists in the
tissues of animals and cells of all kinds of microorganisms. LDH is a large sulfhydryl oligomeric
enzyme consisting of four subunits [75]. These subunits can be classifi ed into two types, that is,
M type and H type. These two types can be assembled into fi ve tetramers of H4, H3M, H2M2, HM3,
and M4. Therefore, there are fi ve isoenzymes of LDH, expressed as LDH1, LDH2, LDH3, LDH4,
and LDH5, respectively. Though the molecular structures and physicochemical properties of such
fi ve enzymes are different from each other, they catalyze the same reversible conversion between
lactate and pyruvate, with concomitant redox between the cofactor NAD
+
and nicotinamide ade-
nine dinucleotide (NADH) (14.16).
LDH
Lactate
+
NAD
+
pyruvate
+
NADH
(14.16)
Both sides of this reversible reaction can induce the electron transfer in the presence of some
mediators (Med). For example, when lactate is oxidized by NAD
+
with the catalysis of LDH, the