Biomedical Engineering Reference
In-Depth Information
PPy fi lms doped with large alkyl sulfonates is presented in Section 13.4 in which the implications
of the synthesis parameters of PPy nanowires on polymer morphology are examined with x-ray
diffraction.
13.2.2.6
Time Response of the Polypyrrole Films
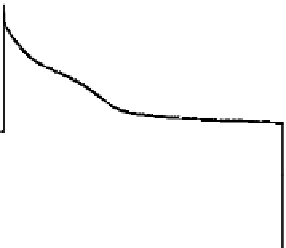
A PPy-fi lm chronoamperogram, or current response to applied voltage steps plotted versus time, is
shown in Figure 13.6.
The experimental setup used to perform chronoamperometry is the same as the one described
above for cyclic voltammetry. A defi ned voltage is applied between the WE and the REF while
current is measured between the WE and the CE. WE is kept at 0 V prior to the start of the experi-
ment, reducing voltage of
−
1 V versus Ag/AgCl is applied at
t
=
0 s, and an oxidizing voltage of
60 s. In Figure 13.6, the current response to the voltage steps
can be observed: fi rst, there is a large current spike that corresponds to charging of the double-layer
capacitance, and then the current begins to decay as the polymer becomes reduced or oxidized,
and ions diffuse in and out of the polymer matrix, respectively. However, the time response of the
current is not purely due to ion diffusion. An infl ection point can be seen clearly in the reducing
current, and the oxidizing current is not a simple exponential decay that could be expected as the
remaining ions leave the polymer by diffusion. The change in conformation of PPy chains, which
occurs during both reduction and oxidation, introduces further time delay into the course of the
current response.
8
In the oxidized PPy, polymer chains are in a “compacted” conformation, packed
close together as there is neither water nor sodium ions in the polymer matrix. When a reducing
voltage is applied, the chains take some time to rearrange their conformation state, which is likely
to be driven by electrostatic repulsion of the dopant DBS
−
ions whose negative charge is no longer
being compensated by the positive charge on the polymer. As the PPy chains move further apart,
the structure of the fi lm becomes less dense, allowing ion and water diffusion into the polymer. The
current-time response is, therefore, a summation of two processes—the conformational changes of
the PPy chains in response to applied voltage and the diffusion of sodium ions and water molecules
to maintain charge neutrality. These two processes combine to produce the infl ection in the mea-
sured current at the point where the fi lm structure becomes fully open and ion diffusion takes over
as the current-limiting factor.
A more quantitative analysis of the PPy electrochemical time response is impeded and
complicated by the presence of other processes that take place during the reversible redox reaction:
changes in conductivity of the PPy fi lm as it passes from an oxidized to a reduced state, current due
0 V versus Ag/AgCl is applied at
t
=
1.2
1.0
0.8
0.6
0.4
0.2
0.0
−
0.2
−
0.4
−
0.6
−
0.8
1.0
−
0
20
40
60
80
100
120
Time (s)
FIGURE 13.6
Chronoamperogram of a large area (
>
1 cm
2
) 30 µm thick polypyrrole fi lm.