Biomedical Engineering Reference
In-Depth Information
the milestone innovations of the mesoporous materials research is the preparation of mesoporous
carbon [77-79]. Ryoo et al. realized this opportunity; they synthesized ordered mesoporous carbon
CMK-1 using cubic MCM-48 silica as template, and sucrose as the carbon source [80]. The fi rst
ordered mesoporous carbon (CMK-3) that was a faithful replica of the template was synthesized
using hexagonal SBA-15 as a template [81]. Mesoporous materials composed of materials other
than silica and carbon have also been widely researched [82,83].
As described in the next section, mesoporous materials have powerful capability in protein
hybridization, but these materials are also highly useful for immobilization of small biomolecules.
Vinu et al. recently reported adsorption behavior of small hydrophobic amino acid and histidine onto
mesoporous materials, and demonstrated superior adsorption capability of mesoporous carbon over
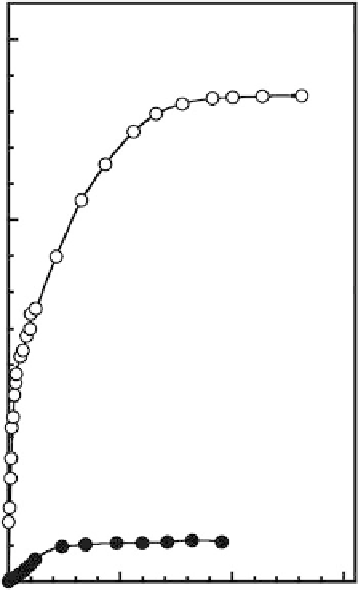
mesoporous silica [84,85]. Figure 12.12 shows the adsorption isotherms of histidine onto mesoporous
carbon CMK-3 (curve (a)) and mesoporous silica SBA-15 (curve (b)) at pH 7.5 (near the isoelectric
point of histidine). Although CMK-3 has a higher mesopore volume and ultralarge pore diameter as
compared with SBA-15, the histidine adsorption capacity observed for CMK-3 is quite high. Superior
adsorption capacity to hydrophobic amino acid histidine would be observed for hydrophobic mes-
oporous carbon. The nitrogen adsorption measurement of CMK-3 adsorbent before and after the
histidine adsorption revealed that the mesopore volume and the surface area of CMK- 3 decreased
drastically after histidine adsorption. For example, upon loading with 1350 mol g
-
1
of histidine, the
specifi c surface area of CMK-3 was reduced from 1260 to 556 m
2
g
-
1
corresponding to 55.9% reduc-
tion of the total surface area, and the specifi c pore volume was reduced from 1.1 to 0.56 cm
3
g
-
1
(49.1%). The large reduction observed in the specifi c pore volume and the specifi c surface area is
attributed to the tight packing of histidine molecule in the mesopores of CMK-3. Such superiority
1500
(a)
1000
500
(b)
0
0
100 200
Histidine (mM)
300
FIGURE 12.12
Adsorption isotherms of histidine onto mesoporous materials at pH 7.5: (a) CMK-3 and
(b) SBA-15.