Biomedical Engineering Reference
In-Depth Information
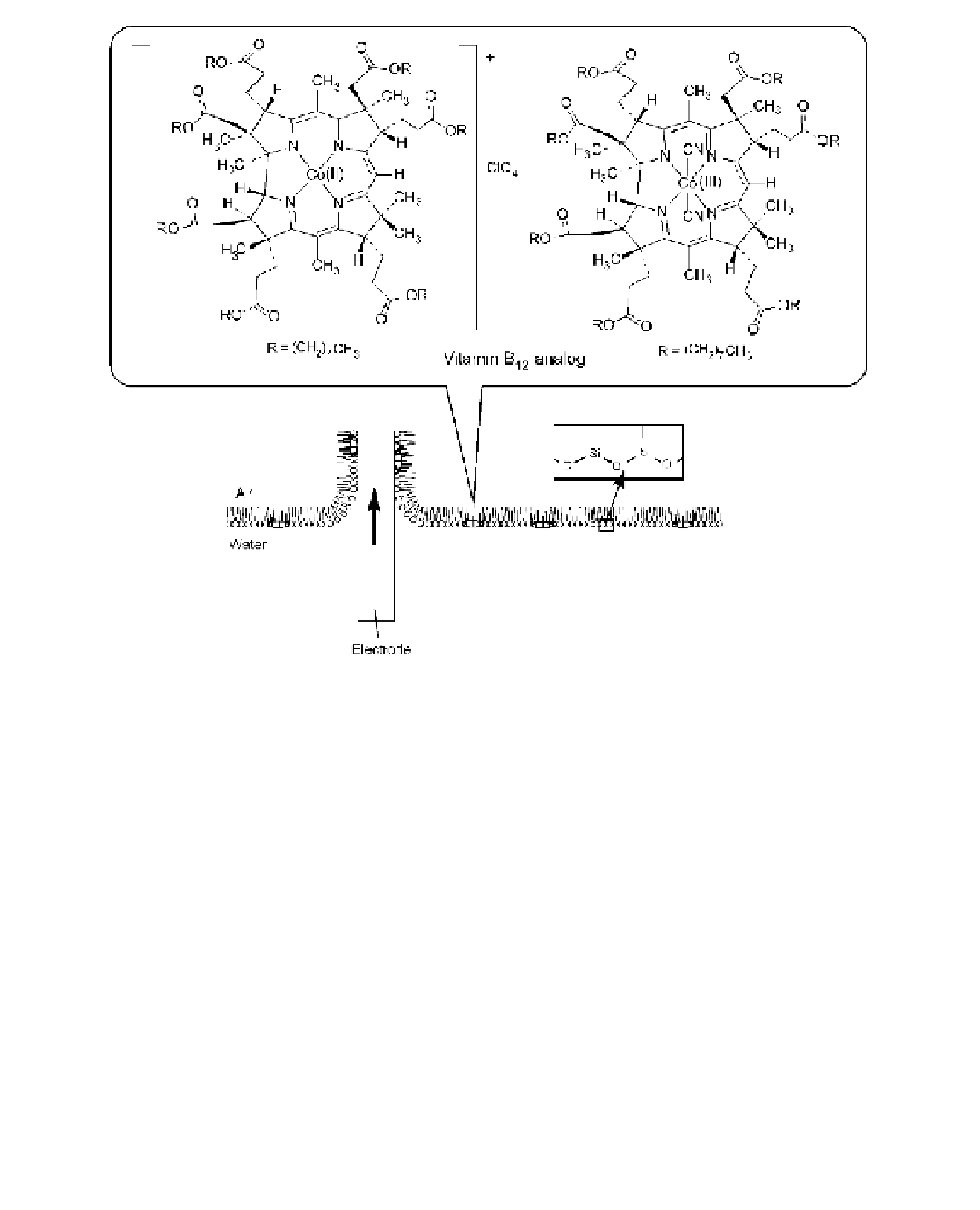
FIGURE 12.11
Incorporation of vitamin B
12
mimics in silica-supported monolayer and its transfer onto an
electrode.
was structurally identical with naturally occurring vitamin B
12
and seven ester chains on the outside
was spread as a mixed monolayer with a dialkylorganosilane amphiphile. The mixed monolayer
containing the vitamin B
12
function was immobilized on an indium tin oxide (ITO) electrode. In a
cyclic voltammetric response of the prepared electrode in aqueous solution, a Co(II)/Co(I) redox
couple at
0.65 V versus Ag/AgCl was observed, which was in good agreement with the corre-
sponding value of the vitamin B
12
derivative dissolved in methanol. This modifi ed electrode would
be used as the reactive electrode with a vitamin B
12
function.
In practical application, porous materials would be useful supports for hybridization of
biocomponents, because huge surface area and pore volumes are advantageous for facile and effec-
tive interaction with external guests. Materials with regularly arranged pores with diameters in
the range 2-50 nm are considered mesoporous materials. The pore sizes of mesoporous mate-
rials are capable of accommodating a wide range of biomolecules, from small molecules such
as amino acids to biopolymers such as proteins. In 1990, Kuroda and coworkers fi rst reported
the preparation of mesoporous silica (FSM-16) with a uniform pore size through the intercala-
tion of cetyltrimethylammonium cations into the layered polysilicate kanemite [60,61]. A signifi -
cant breakthrough in mesoporous materials research occurred when Mobil scientists synthesized
the M41S family of silicate oraluminosilicate mesoporous molecular sieves, including MCM-41
[62,63]. Various kinds of mesoporous silica materials named as SBA, HMS, MSU, MCF, and others
have been synthesized by continuous research efforts in the corresponding fi elds [64-76]. One of
−