Biomedical Engineering Reference
In-Depth Information
shear damage. Electrospraying technique has recently been investigated as a method for preparing
encapsulated particles and beads in a controllable size. The encapsulated substances can be imbed-
ded into the microparticles by mixing the living cells or biological materials with the precursor or
by reacting to the electrosprayed droplets with a collecting solution to form microbeads.
11. 2. 8 .4 .1
Biomolecule-Encapsulated Biomaterials
Amsden et al. has studied electrospraying for generating polymeric microbeads loaded with solid
protein particles [99]. This method involved electrospraying a suspension of protein particles within
a polymer solution under an electric fi eld. The electric force effectively atomized the droplet off the
end of the needle and generated a series of smaller droplets. The electrosprayed droplets formed
microbeads by reacting with a collecting solution. In the work, a solution of BSA particles sus-
pended in a mixture of ethylene vinyl acetate (EVA) and dichloromethane was used for electro-
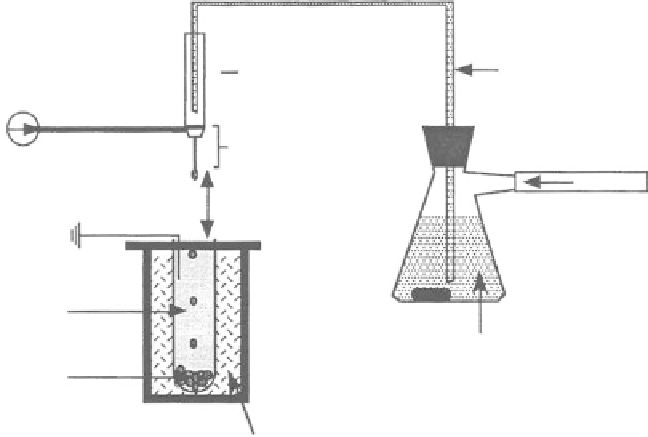
spraying. Figure 11.42 shows the experimental setup for the microbead production. The mixed
solution was poured into a stirring sealed fl ask. The agitated protein and dissolved EVA suspension
was forced through a needle by air pressure, which was regulated to ensure that the fl ow rate of
the suspension was kept at 0.5 mL/min. A positive electrode was connected to the needle while a
ground was applied to the collecting solution, which was 4 cm below the needle. The collecting
solution consisted of methanol cooled to
75°C using a dry ice/methanol bath contained in a Dewar
fl ask so that the EVA promptly gelled and then slowly precipitated upon entering the solution. The
electrosprayed EVA/protein microbeads were kept in the cold methanol for 5 min and then trans-
ferred along with the methanol into a glass dish, which was stored in a
−
20°C environment for
2 days. After that, the microbeads were placed in a vacuum at room temperature for 1 day to remove
the solvent completely. The experimental results illustrated that the microbead size and size distri-
bution were controlled primarily by the strength of the electric fi eld, the gauge of the needle, and the
EVA concentration. Figure 11.43 shows a diagram of the electrosprayed bead diameter distributions
using a 15% w/v EVA concentration and an applied voltage of 4.0 kV. The smaller microbeads can
be formed by using a higher electric fi eld and a smaller internal diameter needle. Protein was effec-
tively incorporated into the microbeads, which was demonstrated by its release from the microbeads
into a saline solution. The critical volumetric loading for the microbeads was dependent on the size
−
Glass tube
Glass syringe
Positive
electrode
Needle
Air
4.0 cm
Ground
MeOH (
−
70
°
C)
EVA in CH
2
Cl
2
+ protein particles
Microbeads
Dry ice and MeOH
bath
FIGURE 11.42
A schematic diagram of microbead production electrospraying apparatus. (Reprinted from
Amsden, B.G. and Goosen, M.F.A.,
J. Contr. Release
, 43, 183, 1997. © Elsevier Science. With permission.)