Biomedical Engineering Reference
In-Depth Information
(c)
(a)
(b)
Cone
Spray
250
100
m
−
µ
100
µ
m
100
µ
m
FIGURE 11.40
Schematic diagram of the cone-jet electrospraying mode (a), electrospraying stops due to an
increase of the distance between the tip and substrate (b), and electrospraying resumes (c). (Reprinted from
Moerman, R. et al.,
Anal. Chem
., 73, 2183, 2001. © American Chemical Society. With permission.)
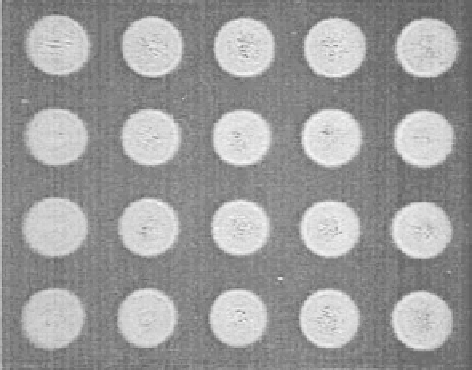
FIGURE 11.41
Images of protein spots manufactured by miniaturized electrospraying. Spot diameter—
260 µm. (Reprinted from Moerman, R. et al.,
Anal. Chem
., 73, 2183, 2001. © American Chemical Society.
With permission.)
0.5 wt.% of Brij 35, and 0.05 M triethanolamine (TEA). A spraying time of 1 s and a spraying
voltage of 1.3 kV were used. The effects of electrospraying on the activities of the relatively labile
enzymes LDH, glucose-6-phosphate dehydrogenase (G6P-DH), and pyruvate kinase (PK) were ana-
lyzed in a quantitative manner. The experiment revealed that electrospraying of LDH, G6P-DH, and
PK on a liquid layer resulted in a complete preservation of their activities. Particularly, when multiple
analytes need to be studied simultaneously and quantitatively, a biochip device is needed to allow for
parallel dispensing of small liquid volumes accurately without cross-contamination. A miniaturized
electrospraying in the cone-jet mode was proved to be a powerful and promising technique for manu-
facturing biochip microarrays of active enzyme spots. The biological activities can be completely
preserved after electrospraying at the moderate processing parameters with an appropriate solution.
11.2.8.4
Encapsulated Electrospraying of Biomaterials
Encapsulation technology for preparing microbeads and microcapsules has been used in many
fi elds [94-98]. For example, microparticles encapsulated with cells were applied for cell trans-
plantation therapy in medicine for the treatment of several diseases and for protecting cells from