Biomedical Engineering Reference
In-Depth Information
demands is getting wider. How to effi ciently deliver these protein agents has a direct impact on
the potential of protein therapy. Challenges in protein delivery have long been recognized because
of the marginal stability of proteins. Unlike low-molecular weight drugs, they possess secondary,
tertiary, and, in some cases, quaternary structures with labile bonds and side chains with chemi-
cally reactive groups [111]. These structures can be easily modifi ed and even destructed. How to
store and deliver these biomacromolecules to target tissues without loss of their functions is a key
question that needs to be answered. In addition, we have to consider unfavorable factors exist-
ing in conventional administration routes, including subcutaneous, intramuscular, intravenous,
inhalation, and oral.
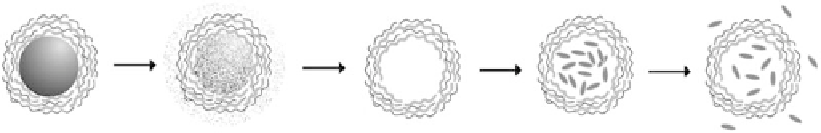
Based on this microencapsulation and further removal of templates (Figure 10.5), a unique
micro/nanocarrier system “hollow shells” was fi rst developed in 1998 [50,112,113]. After
multilayered polyelectrolytes are adsorbed on templates, the core can be removed mainly by
short-time exposure to acids. The capsules are monodisperse in size and the capsule wall serves as
a permeable barrier. Ligands, enzymes, and inorganic nanoparticles can be further assembled on
shell surface for targeting, bioreaction, and other applications. Polyelectrolyte shells are potential
candidates for drug and DNA delivery and may be applied for biosensing when loaded with
molecular probes. The interior environment of a polyelectrolyte shell is aqueous, similar to that
of liposome and polymer vesicles. Obviously, hydrophilic materials are favorable candidates for
loading. In comparison, the hydrophobic core of a polymer micelle is good for encapsulation of
hydrophobic drugs or diagnostic agents [114,115]. The advantages of hollow polyelectrolyte shells
are as follows: (1) a capsule diameter can be varied from tens of nanometers to tens of microns
based on the choice of template; (2) a wide range of sacrifi cial templates including weakly cross-
linked melamine-formaldehyde (MF) particles [50], organic [116] and inorganic crystals [117],
metal nanoparticles [17], and biological templates [118] are available; (3) shell materials are not
only limited to polymers; other charged materials such as inorganic nanoparticles, lipids, and
proteins can also be used; (4) the shell interior environment such as pH value can be adjusted to
be different from exterior conditions; (5) the shell wall permeability can be controlled by shell
materials and shell thickness; and (6) engineered shells can be responsive to external signals such
as low-frequency alternating magnetic fi eld for triggered release of loaded materials [119].
LbL self-assembled hollow polyelectrolyte shells have been studied for encapsulation of
different proteins including α-chymotrypsin [120,121], peroxidase [122-124], urease [29], bovine
serum albumin (BSA) [125-127], oligonucleotide [128], and insulin [129]. Shells are usually in
micron size and subject to “open” and “close” under changes of environmental pH, temperature,
solvent, and even magnetic fi eld (Figure 10.5). Basically, shells are switched to “open” in one condi-
tion for protein loading and then to “close” for storage. It is important to choose the “close” state
similar to the physiological conditions, so proteins will stay inside capsules for long-term storage
with minimum initial leakage during administration. Protein release can be triggered passively by
pathological conditions or actively by external signals. For example, when capsules are accumulated
at tumor site, the low-pH environment [130] would be helpful to passively trigger protein release.
We will discuss how to load and release proteins through pH, external magnetic fi eld, and porous
particle templates.
Core
dissolution
Purification
Loading
Releasing
FIGURE 10.5
Scheme of fabrication of functional hollow polyelectrolyte shells and loading of biomacro-
molecules.