Biomedical Engineering Reference
In-Depth Information
a
c
200 nm
b
100 nm
200 nm
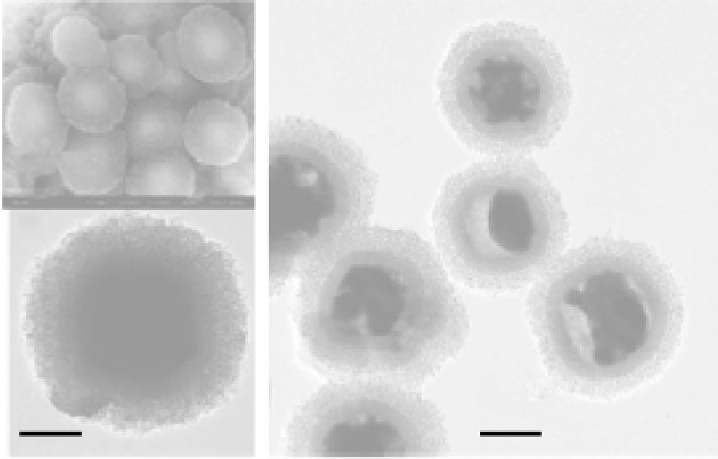
FIGURE 9.8
Backscattered electrons image (a) and TEM micrograph (b) of spheres with a hematite core/
mesoporous silica shell structure, and TEM micrograph (c) of spheres with a Fe
3
O
4
/Fe core/mesoporous
silica shell structure. (Reprinted from Zhao, W.R. et al.,
J. Am. Chem. Soc.
, 127, 8916, 2005. © American
Chemical Society.)
Fu et al. [53] reported the preparation of microspheres with a core-shell structure. The mag-
netite nanoparticles were synthesized by a chemical coprecipitation method using ferrous and
ferric salts, followed by activation treatment with trisodium citrate. The magnetite nanoparticles
were coated by a silica layer, and the magnetic silica nanoparticles (about 30 nm in diameter) were
labeled with fl uorescein isothiocyanate (FITC). After incorporation of the FITC molecules into
the silica layer of the microspheres, another silica layer on these spheres was needed to avoid the
aggregation as a result of the introduction of the FITC molecules. Then, the shell of cross-linked
poly(
N
-isopropylacrylamide) (PNIPAM) was formed on these spheres (about 200 nm). The exper-
iments indicated that these microspheres exhibited multistimuli-responsive properties, offering
promising applications in controlled drug delivery.
Lin et al. [54] reported the synthesis of a controlled-release delivery carrier based on MCM-
41-type mesoporous silica nanorods (MSNs) capped with superparamagnetic iron oxide nanoparti-
cles (Figure 9.9). The system consisted of MSNs functionalized with 3-(propyldisulfanyl) propionic
acid to provide “linker-MSNs,” which had a diameter of 80 nm and length of 200 nm and an average
pore diameter of about 3 nm. Fluorescein was used as the guest molecule to be encapsulated inside
the linker-MSN. By introducing dry linker-MSNs to an aqueous solution of fl uorescein, the meso-
pores of the MSNs behaved like sponges, soaking up fl uorescein molecules. Then, the openings of
the mesopores of the fl uorescein-loaded linker-MSN were covalently capped
in situ
through ami-
dation of the 3-(propyldisulfanyl)propionic acid functional groups bound at the pore surface with
3-aminopropyltriethoxysilyl-functionalized superparamagnetic iron oxide (APTS-Fe
3
O
4
) nanopar-
ticles. The disulfi de linkages between the MSNs and the Fe
3
O
4
nanoparticles were chemically labile
and could be cleaved with various cell-produced antioxidants and disulfi de reducing agents such as
dihydrolipoic acid (DHLA) and dithiothreitol (DTT), respectively.
The experiments showed that less than 1.0% fl uorescein in magnet-MSNs were released in the
PBS solution (0.1 M, pH 7.4) over a period of 132 h in the absence of trigger molecules, indicating
a good effi ciency of the Fe
3
O
4
nanoparticles to retain fl uorescein molecules and prevent undesired