Biomedical Engineering Reference
In-Depth Information
activity or changes in physiological conditions such as pH, osmolality, or temperature, leading
to increased uptake of the drug by the tumor cells at the target sites [47]. Alexiou et al. [48]
investigated the distribution of the magnetic carrier at the cellular level
in vitro
and that of the
chemotherapeutic agent (i.e., mitoxantrone)
in vivo
.
In vivo
experiments were performed in VX2
tumor-bearing rabbits using magnetic nanoparticles bound to mitoxantrone. The researchers found
an increasing concentration of the chemotherapeutic agent in the tumor region after magnetic drug
targeting compared with regular systemic chemotherapy.
The magnetic nanostructures used for drug delivery systems are usually iron oxides. Recent
reports have demonstrated the feasibility of surface-functionalized, superparamagnetic iron oxide
nanoparticles for use in a variety of biological applications. Most of these systems comprise mag-
netic nanoparticle “cores” coated with organic or inorganic “shell,” and the pharmaceutical drugs
are encapsulated within the layers of these shells. The magnetic nanostructures usually need to be
coated with a polymer or an inorganic shell to make them biocompatible and prevent their aggrega-
tion. Moreover, the coating surface of the magnetic nanoparticles can be functionalized to enable
the binding of drugs or biomolecules to the system [49-51]. Silica can be used as the inorganic shell
for magnetic nanoparticles. Amorphous silica is biocompatible, nontoxic, and possesses hydroxyl
surface groups that provide intrinsic hydrophilicity and enable surface attachment by covalent link-
ages of specifi c drugs or biomolecules. Amorphous silica is also a heat-resisting material, with a
low specifi c gravity, high surface area, and good mechanical strength. The small pore size of silica
can selectively interact with the adsorbed molecules depending on their size, shape, and chemi-
cal characteristics. In the case of drug delivery applications, the drug release rate is slow from the
mesopores of the amorphous silica and can be controlled by tailoring the pore size distribution and
thickness of the coating as a function of the drug characteristics, which is advantageous compared
with polymer-coated drug delivery systems in which a faster drug release often takes place.
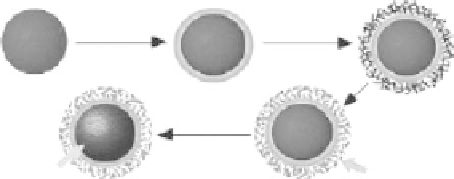
Shi et al. [52] reported the preparation of spheres with a Fe
3
O
4
/Fe core/mesoporous silica shell
structure. Hematite nanoparticles were fi rst prepared as the initial cores, then a thin mesoporous
silica layer was deposited on the surface of hematite nanoparticles. The mesoporous silica shell
was formed from simultaneous sol-gel polymerization of TEOS and
n
-octadecyltrimethoxysilane
(C18TMS) followed by removal of the organic template through calcination. Finally, the hematite
cores of the spheres were reduced in a fl owing gas mixture of H
2
and N
2
to produce Fe
3
O
4
/Fe
(Figure 9.7). The thickness of the silica shell could be tuned from 10 to 50 nm, and the core/shell
spheres had diameters of ca. 270 nm (Figure 9.8). The room temperature magnetic measurement
showed a magnetic hysteresis loop with a saturation magnetization of 27.3 emu/g, indicating the
strong magnetic response to a magnetic fi eld. The drug loading and release property of the core/
shell spheres were also investigated. The uptake amount of IBU was ca. 12 wt.%. The release rate
of IBU in a simulated body fl uid (SBF) was relatively fast during the fi rst 24 h, but decreased with
time and reached a value of 87% after 70 h.
TEOS
TEOS/C18TMS
Hematite
Calcined
Reduced
Mesoporous silica
shell
Magnetic
core
FIGURE 9.7
Illustration of the synthetic strategy of spheres with a Fe
3
O
4
/Fe core/mesoporous silica shell
structure. (Reprinted from Zhao, W.R. et al.,
J. Am. Chem. Soc.
, 127, 8916, 2005. © American Chemical
Society.)