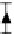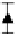Biomedical Engineering Reference
In-Depth Information
0.35
Cumulative glucose release (mg)
Glucose release rate (mg / days)
0.3
0.25
0.2
0.15
0.1
0.05
0
0
1
2
3
4
5
6
Time (days)
FIGURE 9.6
Glucose release profi le through 60 nm channel. (Reprinted from Sinha, P.M. et al.,
Nanotech-
nology
, 15, S585, 2004.)
of drugs. Glucose released through such nanochannels (60 nm) validated the zero-order release
profi le. Figure 9.6 shows the release profi le of glucose over a 5-day period. A zero-order release
profi le was achieved within the experimental error, enabling maintenance of drug delivery through
the nanochannels in a therapeutic window.
9.3 NANOSTRUCTURED CALCIUM CARBONATE
AND CALCIUM PHOSPHATES AS DRUG CARRIERS
Calcium carbonate (CaCO
3
) and calcium phosphates (
β
-Ca
3
(PO
4
)
2
, hydroxyapatite (Ca
10
(PO
4
)
6
(OH)
2
,
HA), etc.) are common biominerals. These biominerals possess advantages in applications as drug
carriers because of their biocompatibility, nontoxicity, and good biodegradability.
Mizushima et al. [33] reported a simple method for incorporating drugs (betamethasone phos-
phate and erythropoietin) into CaCO
3
nanoparticles.
In vitro
release test showed that granulocyte
colony-stimulating factor incorporated in CaCO
3
nanoparticles was chemically stable and released
very slowly. Subcutaneous injection of CaCO
3
nanoparticles incorporating betamethasone phos-
phate resulted in a smaller initial increase in plasma concentration and a subsequent sustained
release compared with that in betamethasone phosphate solution.
Tong et al. [34] reported the preparation of porous CaCO
3
microspheres with an average diam-
eter of 5 µm, which were used as the drug (IBU) carrier. The microspheres looked very rough on
surface morphology and consisted of nanoparticles and channel pores with sizes of about 20 nm. The
adsorbed IBU amount was 45.1 mg/g for one-time adsorption and increased with increasing adsorp-
tion times. Finally, multilayer fi lms of protamine sulfate and sodium poly(styrene sulfonate) were
formed on the IBU-loaded CaCO
3
microspheres by the layer-by-layer self-assembly. IBU-loaded
CaCO
3
microspheres had a rapid release in the gastric fl uid and a slower release in the intestinal fl uid
compared with the bare IBU crystals. Polyelectrolyte multilayers capped on the drug-loaded spheres
reduced the release rate in both the fl uids. In the simulated gastric fl uid (pH 1.2), the half-release time
was 180, 70, and 100 min for the IBU crystals, IBU-loaded CaCO
3
microspheres, and IBU-loaded
microcapsules, respectively. The total release time for the corresponding spheres was 500, 250, and
500 min. In the simulated intestinal fl uid (pH 7.4), the half-release time was 12, 25, and 60 min for
the IBU crystals, IBU-loaded CaCO
3
microspheres, and IBU-loaded microcapsules, respectively,
and the corresponding total release time was 150, 230, and 320 min. In addition, Ueno et al. [35]
investigated nanosized CaCO
3
used as a carrier for sustained release of betamethasone phosphate.