Biomedical Engineering Reference
In-Depth Information
into three sections: (1) nanostructured silica as drug carriers, (2) nanostructured calcium carbonate
and calcium phosphates as drug carriers, and (3) magnetic targeting drug delivery systems.
9.2 NANOSTRUCTURED SILICA AS DRUG CARRIERS
Some papers have been publ ished on t he d r ug ca r r iers of SiO
2
nanoparticles. Barbé et al. [6] reported
that bioactive molecules could be encapsulated within silica nanoparticles by combining sol-gel
polymerization with either spray-drying or emulsion chemistry. Preliminary
in vivo
experiments
revealed the enhanced blood stability of the nanoparticles and the sustained release of antitumor
agents, indicating good potential for cancer treatment (Figure 9.1).
Maitra et al. [7] prepared and characterized hydrated silica nanoparticles encapsulating high
molecular weight compounds such as [
125
I]tyraminylinulin (mol. wt. 5 kDa), FITC-dextran (mol. wt.
19.6 kDa), and horse radish peroxidase (mol. wt. 40 kDa). The entrapment effi ciency was found to
be as high as 80%, and the entrapped compounds showed practically zero leachability for more
than 45 days. Enzymes entrapped in these nanoparticles demonstrated Michaelis-Menten kinetics.
Peroxidase entrapped in silica nanoparticles showed higher stability toward temperature and pH
changes compared with free enzyme molecules.
In recent years, there has been an increasing interest in mesoporous silica materials for their
application in controlled drug release because of their nontoxicity, adjustable pore diameter, high
specifi c surface area, and abundant Si-OH bonds on the pore surface and intrinsic hydrophilicity
and biocompatibility [8-19]. Many research activities have focused on mesoporous silica, both solid
spheres and hollow spheres. These systems exhibit sustained drug release behaviors.
The experimental results show that factors such as crystal structure, pore size, porosity, specifi c
surface area, and functionalization of the pore wall infl uence the controlled delivery of different
drugs. Functionalization is an important infl uencing factor in the drug adsorption and release rate.
It is very important to carefully choose the type of functionalization of the pore wall in agreement
with the specifi c drug to be adsorbed and subsequently released [18].
1
0.8
O
OH
OH
0.6
C
H
2
OH
OH
0.4
H
O
O
OH
O
CH
3
0.2
CH
3
O
NH
2
OH
0
15
20
25
30
0
5
10
Release time (days)
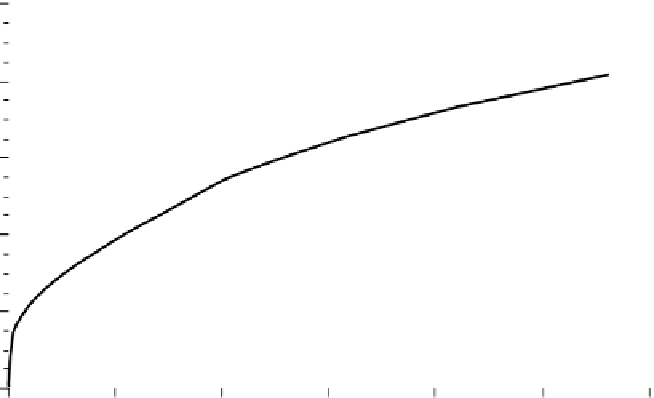
FIGURE 9.1
Release kinetics at pH 6.5-7 for doxorubicin encapsulated in 30 nm SiO
2
nanoparticles.
(Reprinted from Barbé, C. et al.,
Adv. Mater.
, 16, 1959, 2004.)