Biology Reference
In-Depth Information
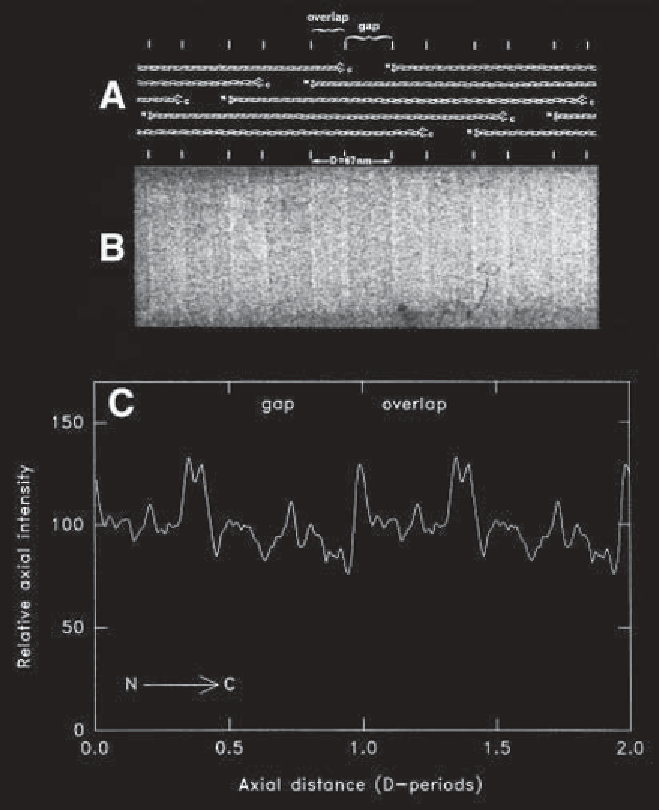
Fig. 1.
(A)
Diagram to show the axial packing arrangement of molecules in the
collagen fibril. The fibrils have an axial periodicity of 67 nm and characteristic gap-
overlap regions. The short nonhelical domains (telopeptides) at the ends of the mol-
ecules are shown. These telopeptides possess an axially contracted conformation
relative to the main triple helical part of the collagen molecule.
(B)
Energy-filtered
TEM image of a collagen fibril from bovine skin in vitreous ice. Defocus is at 1.5
m
to enhance phase contrast. The image has been aligned with the axial packing diagram
(A)
. The gap-overlap structure is apparent and the higher density telopeptide domains
can be clearly distinguished.
(C)
Average axially projected structure of the collagen
fibril in vitreous ice. This has been measured from images similar to
(B)
and is shown
repeated over two
D
-periods.
µ

Search WWH ::

Custom Search