Biology Reference
In-Depth Information
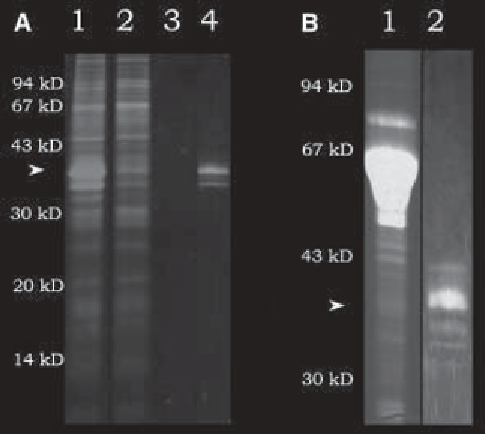
β
2
chain of trans-
fected 293-EBNA cells. The protein carried an N-terminal Strep II tag and was
chromatographed on a StrepTactin column as described in the text. The product occurs
in different glycosylation forms.
(A)
Purification from serum-free medium: (lane 1)
cell culture supernatant; (lane 2) flowthrough; (lane 3) wash; (lane 4) eluate.
(B)
Puri-
fication from medium containing 10% FCS: (lane 1) cell-culture supernatant; (lane 2)
eluate. SDS-PAGE was performed on 15% polyacrylamide gels that were stained with
Coomassie brilliant blue R250. All evident bands in the eluate were immunoreactive
with an antibody specific for the laminin
Fig. 2. Affinity purification of the IV domain from the laminin
β
2
chain.
c. Incorrect estimation of the domain borders may lead to an incorrectly folded
and nonsecreted protein.
d. Poor transfection efficiency with the survival of nontransfected cells gives
low levels of the protein in the media. This may be corrected by hard (1:20)
splitting of the cells and this is indicated when many dead cells are seen after
routine passaging.
e. A low plasmid copy number in the 293-EBNA cells may produce low expres-
sion levels. Increasing the amount of puromycin in the selection media two-
or threefold may raise yields of the recombinant protein.
f. The formation of insoluble deposits may occur where the secreted protein
precipitates out of the media or is incorporated into a matrix around the cells
or upon the plate. It is worthwhile immunoblotting the cell extract after the
cells have been removed from the plate by prolonged washing with EDTA/
PBS as well as trying to extract any proteins in the matrix laid on to the plate
by treating it with SDS-PAGE sample buffer.

Search WWH ::

Custom Search