Biology Reference
In-Depth Information
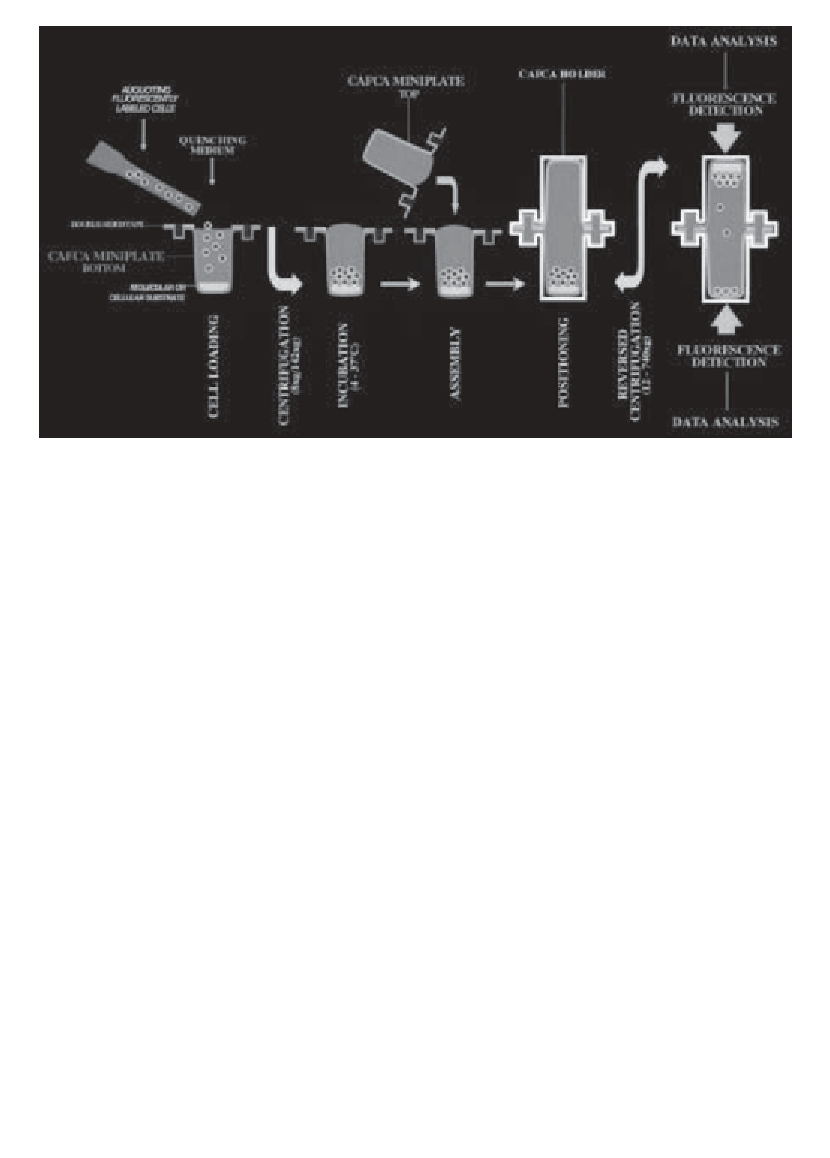
Fig. 1. Schematic representation of the CAFCA procedure.
small number of cells, without limiting the total number of samples/conditions
that can be examined. It is based on the use of a commercially available
microplate fluorometer and a few associated accessories (
Fig. 2
), including,
for maximal convenience, a dedicated software. Finally, as previously sug-
gested for cell-cell adhesion assays
(18-20)
, the employment of vital fluoro-
chromes to label the cells adds to the above advantages that characterize
CAFCA. This is because it renders this procedure an ideal assay for the analy-
sis of freshly isolated normal and diseased human cells.
1.3. Fundamentals of Cell Migration Assays
As hinted above, cell movement, either in the form of a chemotactic/chemo-
kinetic response to soluble factors, or as a phenomenon governed by a
haptotactic response to ECM-associated components, is one of the primary
cellular processes during embryonic development, the maintenance of a healthy
adult organism and the progression of a number of pathological events such as
inflammation and tumor metastasis. Although cell migration has been studied
in numerous in vivo model systems, it has been proven difficult to investigate
the molecular events responsible for the movement of cells and the governing
control mechanisms involved resorting to cell-culture techniques. A variety of
methods have also been described for the studying of cell movement in vitro
and several of these protocols employ different types of 3-dimensional
ECMs
(21-25)
. Cells capable of invading these ECMs can be monitored at
Search WWH ::

Custom Search