Biology Reference
In-Depth Information
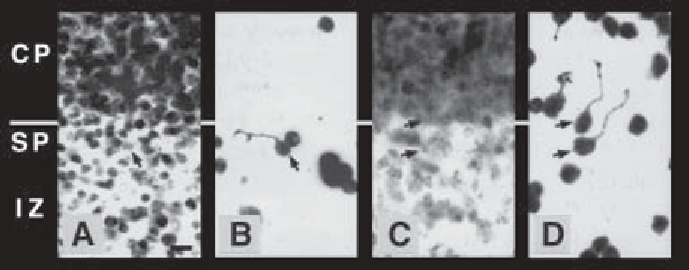
Fig. 2. Cell attachment and neurite extension on slices of embryonic mouse cere-
bral cortex treated with chondroitinase ABC. Cocultures of cortical slices and tha-
lamic neurons were prepared as in
Fig. 1
, except that slice cultures were treated with
carrier (complete medium), or 1 U/mL chondroitinase, for 3 h at 37
C, prior to the
addition of dissociated and labeled thalamic neurons. High-magnification, fluores-
cence micrographs show the bisbenzamide-stained cortical plate (CP), subplate (SP),
and intermediate zone (IZ) of control
(A)
and treated
(C)
tissue slices.
(B,D)
The same
fields viewed under rhodamine optics reveal the attached, thalamic cells and their
neurites. Arrows indicate neurite-bearing thalamic cells and their corresponding nuclei.
In control cocultures
(A,B)
, cells attach well to the subplate, but poorly to the cortical
plate. Neurites that extend on the subplate and intermediate zone tend to orient parallel
with the cortical layers, and neurites that cross from the subplate onto the cortical plate
are extremely rare. In cocultures treated with chondroitinase (C, D), cells attach well
to the cortical plate, neurite outgrowth on the cortical plate is enhanced, and processes
that originate on the subplate often cross onto the cortical plate. Scale bar is 10
°
µ
m.
This chapter will focus on how to cut tissue slices for use as culture substrata
and how to make and fluorescently label a dissociated cell suspension from tis-
sue. It is assumed that the investigator already has procedures to obtain the
tissue(s) of interest. The first four parts of the protocol involve preparing the
slices and dissociated cells for coculture and include: 1. embedding the tissue in
agarose and slicing it on a vibratome, 2. mounting the tissue slices onto sup-
ports and transferring them to culture, 3. dissociating tissue into a cell suspen-
sion, and 4. labeling dissociated cells with a vital dye (
Fig. 3
). The fifth section
deals with culturing, fixing, and counterstaining the cocultures, as well as how to
mount them onto microscope slides for visualization.
The choice of technique for visualizing the cells on the slices (e.g., immuno-
fluorescence,
in situ
hybridization, transgenic marker, etc.) will, of course,
depend upon the experimental needs of the investigator. The method described
below involves labeling the cells in suspension, before plating, with a fluores-
cent, fixable, cytoplasmic dye that makes it easy to discern plated cells from

Search WWH ::

Custom Search