Biology Reference
In-Depth Information
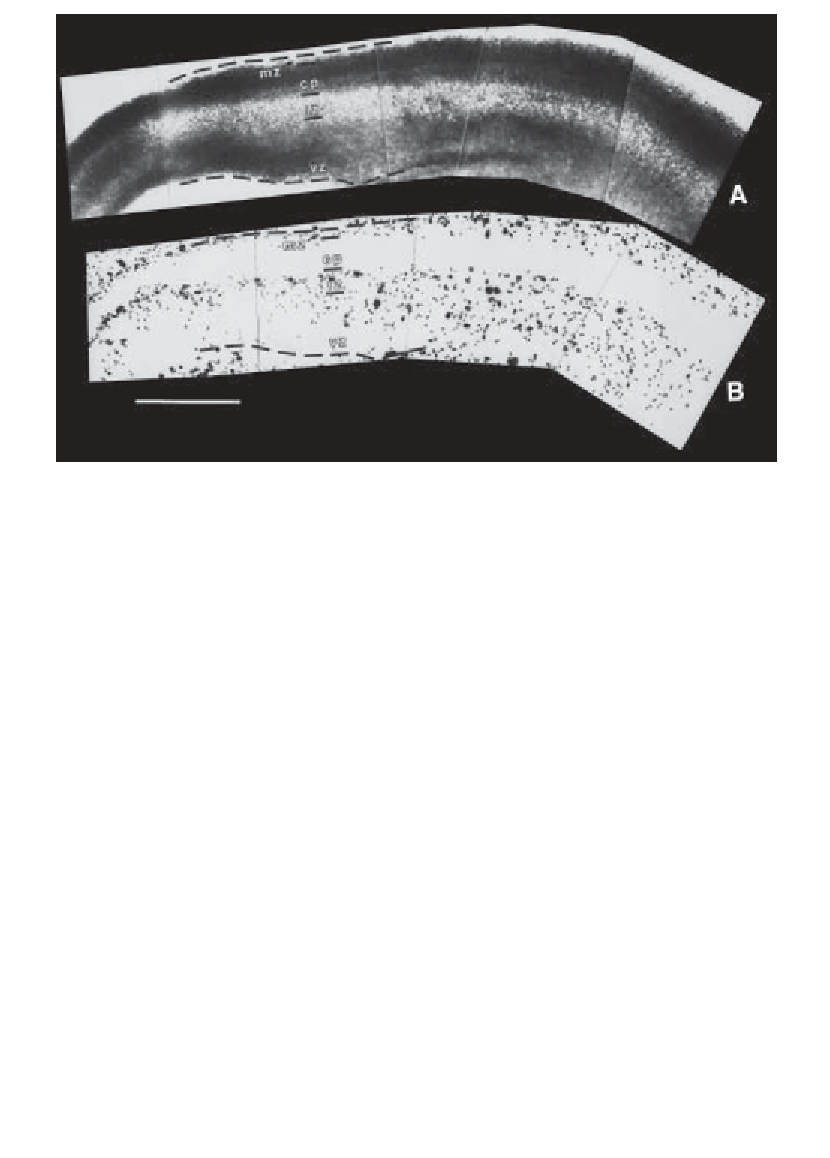
Fig. 1. Laminar specific attachment of thalamic neurons to a living slice of embry-
onic mouse cerebral neocortex. Dissociated and fluorescently labeled embryonic tha-
lamic neurons were plated onto sagittal slices of embryonic day 15 cortex and were
cocultured for 3 h at 37
C. Cocultures were then rinsed to remove nonattached thalamic
cells, fixed, counterstained, and visualized.
(A)
Cortical laminae of a bisbenzamide-
stained slice can be seen under UV fluorescence as differences in nuclear density.
Rostral is to the right, and caudal is to the left.
(B)
The same slice viewed under
rhodamine optics shows the distribution of attached thalamic cells. The pial and ven-
tricular edges of the cortex are demarcated by dotted white lines at the top and bottom
of each figure. Lines are positioned in the two photos to reference the same points in
the two views of the slice. Very few thalamic cells attach to the cortical plate (CP),
whereas more attach to the intermediate zone (IZ), marginal zone (MZ), and ventricu-
lar zone (VZ) as well as to the culture support off the slice (seen at the edges of the
photos). Density of attached cells is greatest on the intermediate zone just subjacent to
the cortical plate (i.e., the subplate). Scale bar is 500
°
µ
m.
The living-slice method of coculture is amenable to the same experimental
manipulations that are performed on typical dissociated cell cultures (e.g., phar-
macological treatment, enzymatic digestions, antibody blockade, etc.). For
example, we used this approach to investigate the functions of chondroitin sul-
fate in neuronal attachment and axon pathfinding. We treated slices with
chondroitinase ABC to enzymatically remove endogenous chondroitin sulfate,
and then compared the behaviors of primary neurons plated on treated and
control slices (
Fig. 2
and
ref.
2
).
Search WWH ::

Custom Search