Biology Reference
In-Depth Information
A
f
A
x
f
B
p
k
a
= 7.8
0
p
k
a
= 9.8
-
1
f
B
-
2
f
A
-
3
p
k
a
= 8.2
-
4
-
5
5
6
7
8
9
10
pH
f
A
x
f
B
B
p
k
a
= 7.8
0
General base
Catalysis yields
General acid
Catalysis
p
k
a
-
1
-
2
f
A
p
k
a
= 7.8
f
B
-
3
-
4
-
5
5
6
7
8
9
10
pH
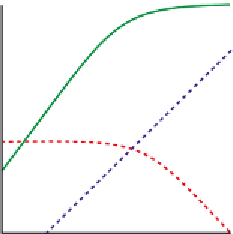
Figure 5.3 Interpretation of glmS ribozyme pH-reactivity profile. (A) By analogy to the
acid
-
base chemistry of the HDV ribozyme, an approximation of the actual pH-reactivity
profile for the glmS ribozyme (green) would require that the contribution of GlcN6P
functioning solely as a general acid catalyst following its solution pK
a
(red) be combined
with the contribution of a general base catalyst with a pK
a
of 10 (blue). Self-cleavage
activity requires a protonated general acid (fraction f
A
) and an unprotonated general
base (fraction f
B
), with the resultant pH-dependent observed rate constant (k
obs
) propor-
tional to the product f
A
f
B
. (B) An alternative interpretation of the glmS ribozyme
pH-reactivity profile. An approximation of the actual pH-reactivity profile for the glmS
ribozyme (green) might represent the combined contributions of both general
base and general acid catalysis by GlcN6P (yellow), where if general base catalysis
precedes and yields general acid catalysis then the reactivity profile is not diminished
above the apparent pK
a
of GlcN6P.
the absence of coenzyme.
6
In addition, the apparent p
K
a
of the ribozyme
reaction does not reflect a pH-averaged value consistent with that of the pro-
posed general acid and general base catalysts. The model additionally pre-
dicts that mutation of G33 to adenosine with a nucleobase p
K
a
of 3.9
should support catalysis at near neutral pH, where the functional form of




































Search WWH ::

Custom Search