Biology Reference
In-Depth Information
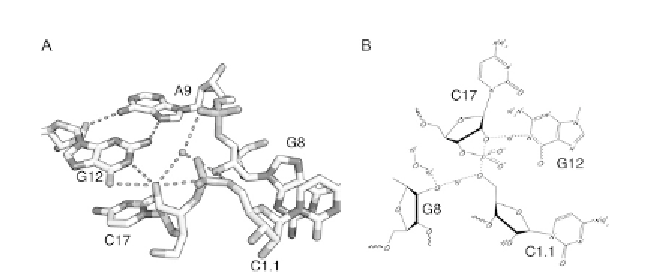
Figure 1.2 The active site of the full-length hammerhead ribozyme permits a mecha-
nism to be proposed. (A) Close-up of the crystal structure of the full-length hammerhead
ribozyme showing G12 positioned for general base catalysis, the 2
0
-OH of G8 poised for
acid catalysis, and the attacking nucleophile, the 2
0
-O of C17, positioned for an in-line
attack upon the adjacent scissile phosphate of C1.1. A9 helps to position G12 and may
also engage in transition-state stabilization of the pentacoordinate oxyphosphorane
transition state. (B) A mechanistic diagram illustrating partial proton dissociation and
transfer in a putative transition state.
positioned in a manner consistent with a role as the general base in the reac-
tion. A transiently deprotonated G12 might then be able to abstract a proton
from the 2
0
-OH, generating the required attacking nucleophile for the
cleavage reaction. The 2
0
-O is pre-positioned for in-line attack, and a second
hydrogen-bonding interaction between the 2
0
OH of G8 and the leaving
group 5
0
-O of C1.1 may represent a general acid catalytic mechanism.
The invariant G8 forms a Watson-Crick base pair with C3, another invari-
ant residue. Mutation of either one of these abrogates ribozyme activity
completely, but a double mutation (i.e., C8/G3) that restores the base pair
restores activity to the hammerhead ribozyme. Thus, it appears that the
ribose of G8 rather than the nucleobase provides the relevant acidic moiety
for catalysis, although other factors are no doubt involved.
3.4. Resolution of experimental discord
Many of the biochemical experiments designed to probe transition-state
interactions and the chemistry of catalysis appeared to be irreconcilable with
the minimal hammerhead crystal structures. For example, the invariant core
residues G5, G8, G12, and C3 in the minimal hammerhead ribozyme were
each observed to be so fragile that changing even a single exocyclic func-
tional group on any one of these nucleotides resulted in abolition of catalytic
activity, yet few of these residues appeared to form hydrogen bonds involv-
ing the Watson-Crick faces of the nucleobases. A particularly striking and
Search WWH ::

Custom Search