Biology Reference
In-Depth Information
Subtrate +
Ribozyme
A756
None
A756G
O S O S O S
Substrat
e
Pr
o
duct
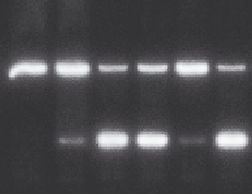
Figure 3.7 Cleavage of the VS substrate by A756 or A756G ribozymes as a function of
the presence or absence of a 5
0
-phosphorothiolate (PS) linkage at the scissile phos-
phate.
65
The 5
0
-O (O) and 5
0
-PS (S) substrates were incubated with no ribozyme (none),
natural sequence ribozyme (A756), or ribozyme in which the critical A756 is replaced by
G (A756G) for 15 min, and the products of cleavage were separated by gel electropho-
resis. Note that cleavage of the oxy substrate is strongly impaired when the A756G ribo-
zyme is used, but that cleavage is restored by the phosphorothiolate substitution. This is
strongly indicative of A756 acting as general acid in the cleavage reaction.
is such a good leaving group that it does not require protonation by a general
acid. However, an H12 mutation was not rescued, consistent with its function
as a general base activating the nucleophile.
We found that the cleavage activity of VS A756G ribozyme was
impaired 1000-fold acting on the oxy (5
0
-PO) substrate, but the activity
was completely restored with the 5
0
-PS-containing substrate (
Fig. 3.7
).
65
The cleavage of the 5
0
-PS substrate thus became insensitive to substitution
at position 756, and we concluded that A756 is therefore the acid. By con-
trast, the rate of cleavage of a G638DAP plus 5
0
-PS substrate was similar to
that observed for a G638DAP plus 5
0
-PO substrate, and both were signifi-
cantly lower than the natural sequence. The pH profile of cleavage rate for
the G638DAP plus 5
0
-PO substrate is bell-shaped, with p
K
a
values of 4.8
and 5.6.
22
However, with the 5
0
-PS substitution the profile changed, with
the reaction rate increasing to pH
6 and remaining at a plateau thereafter.
65
The rate would not be expected to fall at higher pH, if deprotonation of the
acid is no longer relevant. These data were fitted to a single ionization, with a
p
K
a
of 5.3, consistent with general base catalysis by the diaminopurine at
position 638.








Search WWH ::

Custom Search