Environmental Engineering Reference
In-Depth Information
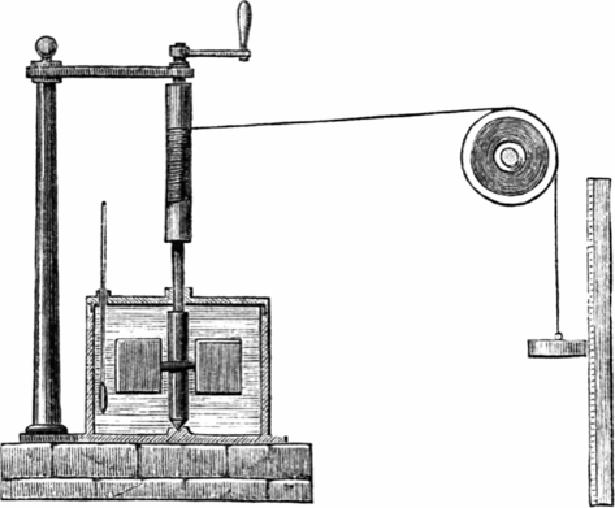
immersedinajarofwater,andheusedaweightandpulleytoturnthebladesofthe'beater'
(see
Figure 1.1
). The movement of the water molecules created heat, which Joule was able
to measure using a thermometer. The greater the weight (force) he used, the faster the
beater turned, and the greater the rise in temperature. In this way, he discovered a simple
yet remarkably accurate way of measuring the relationship between work and heat.
Figure 1.1.
Joule's apparatus for measuring the relationship between work and heat. The
fall of the weight causes the blades to turn, stirring - and thus heating - the water inside
the container (calorimeter). A thermometer measures the rising temperature.
Joule's experiment led to the formulation of one of the most important principles of
physics: the first law of thermodynamics. This states that energy can be neither created
nor destroyed, but merely changed from one form to another. Think of what happens when
a car brakes: the energy of its movement is not lost, just converted into another form of
energy. The brake pads, discs and surrounding air are warmer than they were before the
driver braked. This principle is crucial to understanding how energy can be generated and
used.