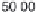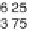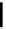Geology Reference
In-Depth Information
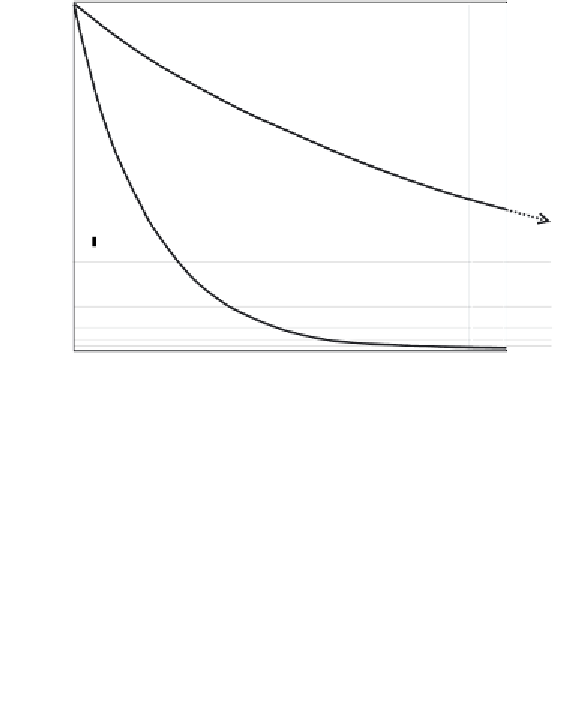
The decay rate of uranium 235, compared with uranium 238.
Today we believe that uranium formed in a supernova more than
6000 million years ago. This material was then inherited by the
solar system, of which the Earth is a part, around 4600 million
years ago. The production ratio of uranium 235 to 238 in a super-
nova is about 1.65, but in 1929 Rutherford assumed they were pro-
duced in equal amounts. From the diagram it can be seen that
uranium 235 decays much faster and so has passed through more
than six of its half lives by the time uranium 238 has decayed to
only half its original amount.
far too much lead, given the amount of uranium or thorium
present, so there must have been some there already when those
elements started to decay. But what was its isotope number? The
question remained unanswered for several years until, in 1936,
a young physicist was invited to join a team at Harvard University
to work with the latest and best designed mass spectrometer.
Although a physicist by background, Alfred Nier soon became
interested in the problems of measuring geological time, and
resolved to try and identify all the known isotopes of lead on his