Chemistry Reference
In-Depth Information
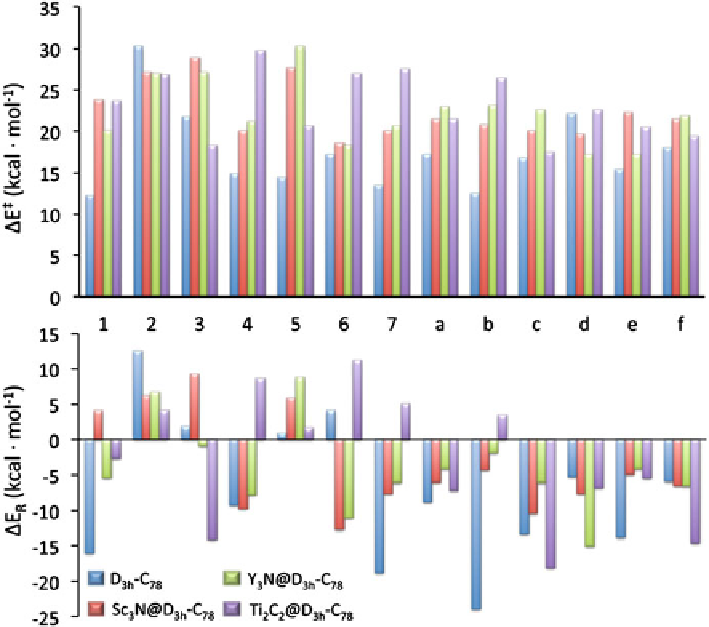
Fig. 4.4
Comparison of the reaction energy barriers and reaction energies (in kcal mol
−
1
) for the
DA reaction over all non-equivalent bonds of X@
D
3h
-C
78
(X
Ø, Sc
3
N, Y
3
N, and Ti
2
C
2
) EMFs.
For Y
3
N@
D
3h
-C
78
only energies for the down region are reported here (see ref. (Osuna et al.
2009a
)). (Reprinted with permission from (Garcia-Borràs et al.
2012a
). Copyright 2012 Wiley)
=
The most reactive bonds for the
D
3h
-C
78
pristine cage are the pyracylenic (type
A) [6, 6] bonds
1
and
7
and the corannulene (type D) [5, 6] bond
b
(for bond
1
:
E
R
=−
16.0 kcal mol
−
1
,
E
‡
12.2 kcal mol
−
1
; for bond
7
:
E
R
=−
=
18.8
kcal mol
−
1
,
E
‡
13.5 kcal mol
−
1
; and for bond
b
:
E
R
=−
23.9 kcal mol
−
1
,
=
E
‡
12.5 kcal mol
−
1
). It is important to remark here that pyracylene bonds are
also the most favorable addition sites for C
60
.
When the scandium based metal cluster is encapsulated, the regioselectivity
changes towards the additions over type B [6, 6] bonds
6
and
4
, and the type D [5, 6]
bond
c
(for bond
6
:
E
R
=−
=
12.7 kcal mol
−
1
,
E
‡
18.5 kcal mol
−
1
; for bond
4
:
=
9.7 kcal mol
−
1
,
E
‡
20.0 kcal mol
−
1
; and for bond
c
:
E
R
=−
E
R
=−
=
10.4
kcal mol
−
1
,
E
‡
20.1 kcal mol
−
1
). And when the yttrium TNT EMF is con-
sidered (Y
3
N@
D
3h
-C
78
, the largest cluster studied), the most favorable additions
are those corresponding to the attack over type D [5, 6] bond
d
(
E
R
=−
=
15.0