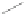Chemistry Reference
In-Depth Information
Fig. 1.7
Clar structures of anthracene, phenanthrene, and triphenylene
651 nm, blue-green
840 nm, deep green
539 nm, red
425 nm, yellow
328 nm, colorless
Fig. 1.8
Heptacatafusene isomers C
30
H
18
with increasing numbers of Clar sextets from 1 to 5:
longest absorption wavelength (in
nanometers
), and corresponding color
stability and many other characters associated with aromaticity are present.
Hückel's
4n
2π-electron rule
as a necessary and sufficient condition for planar aromatic sys-
tems applies also to ionic and to heterocyclic structures. For the simplest benzenoid,
benzene, which has a
+
-electron sextet and is the prototype of perfect aromaticity,
one can write two Kekulé valence structures. For [
k
]acenes with
k
linearly condensed
benzenoid rings one can write
n
π
+
1 Kekulé structures, but for kinked benzenoids
such as phenanthene or branched ones such as triphenylene, the number of Kekulé
structures is higher.
Sir Robert Robinson invented formulas with a circle symbolizing a
-electron
sextet. Eric Clar observed that electronic absorption spectra of benzenoids, which
account for the colors of these hydrocarbons, are correlated with formulas (nowadays
called Clar structures) using the sextet circle under the assumption that in polycyclic
benzenoids there is a tug-of-war between rings trying to own
π
-electron sextets (Clar
1972
). In acenes, the unique Clar sextet can move along and this is symbolized by
arrows, as seen in Fig.
1.7
. Clar formulas must have the maximum possible number
of sextet rings; a ring can either have a sextet, or one or two double bonds; no sextets
can be written in adjacent rings.
In Fig.
1.8
one can see Clar structures of five isomeric heptacatafusenes, starting
with heptacene which absorbs red photons (and is therefore colored deep green,
having
k
π
7 and 1 Clar sextet), and ending with tetrabenzanthracene which has 5
Clar sextets and absorbs only in the ultraviolet region and is therefore colorless.
=