Chemistry Reference
In-Depth Information
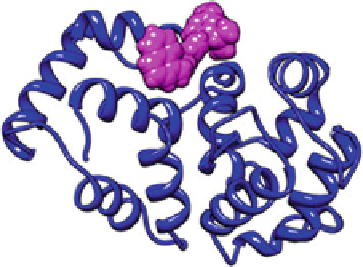
Fig. 9.8
Predicting binding
poses of therphenyl (viewed
as magenta surface) into
bovine recoverin (viewed as
blue
ribbon). There are 57
almost superposed poses
concerning the same region of
the target protein,
magenta
region on the top of figure
Fig. 9.9
The best scored pose
for therphenyl 2 (viewed as
magenta
surface) binding into
bovine recoverin (viewed as
blue
ribbon)
energies are estimated on a grid, (iii) the binding modes with the most favorable
energies are evaluated and clustered and (iv) the most favorable clusters can be
visualized online or/and downloaded by user.
An illustration of using molecular docking technique under SwissDock server to
investigate a protein-ligand interaction is provided here for the putative therphenyl
binding into bovine recoverin. Therphenyl is a small hydrophobic molecule mim-
icking one face of an alpha-helical peptide, i.e. the side chains of three key residues
occupying positions i, i
+
3 and i
+
7 (Fairlie et al.
1998
, Maity and Konig
2008
)
or i, i
7(Yinetal
2005
) of the bound heli9. Terphenyl was found to
interact to other calcium binding proteins: calmodulin (Orner et al.
2001
), centrin
2 (Isvoran et al.
2011
) and troponin C (Isvoran et al.
2013
). The target, i.e. bovine
recoverin, has been prepared as input for docking experiments by using DockPrep
tool of CHIMERA software (Pettersen et al.
2004
) and default parameters of accurate
dock under SwissDock server (Grosdidier et al.
2011
) are considered for calcula-
tions. There are 60 predicted terphenyl binding poses to bovine recoverin (Fig.
9.8
),
57 of them concerning the same region of protein but with distinct orientations of
the ligand. The best scored docking pose is given in Fig.
9.9
.
The predicted interaction energy of the best scored pose of therphenyl 2 to bovine
recoverin is
+
4 and i
+
−
8.96 kcal/mol and it is in very good agreement with the predicted