Biomedical Engineering Reference
In-Depth Information
Fig. 1 Schematic showing
pathways of estrogen and
androgen signaling in the
skeleton with mechanical
loading. (E2: estradiol, the
predominant estrogen; T:
testosterone, the predominant
androgen; ERa: estrogen
receptor-alpha; ERb:
estrogen receptor-beta; AR:
androgen receptor)
of estrogen receptor-a (ERaKO), estrogen receptor-b (ERbKO), and androgen
receptors (ARKO) [
22
].
Loading can be combined with both surgical and genetic models of hormone
deficiency. As described in the previous chapter, increased in vivo loading can
be achieved through exercise (intrinsic) and direct (extrinsic) skeletal loading
Kotiya and Silva). A limited number of studies have examined reduced loading
combined with hormone deficiency. Most of our knowledge comes from
increasing the in vivo loading in hormone-deficient rats and more recently mice;
large animal models of combined exercise and hormone effects are limited [
23
].
In this chapter we will focus on mechanotransduction in rodent models of sex
hormone deficiency.
2 Hormone-Deficiency Induced Models of Osteoporosis
and In Vivo Loading
Sex hormone deficiency results in bone loss and can be induced in preclinical
studies by surgical removal of the gonads to simulate the natural decreases in
hormone production with aging in humans. Preclinical models of surgically
induced hormone deficiency in rodents demonstrate the key features of bone loss
seen clinically [
24
]. Measures to not only counteract but also inhibit this bone loss
and the associated morbidities such as fractures have been studied extensively.
While
pharmacological
treatments
are
currently
the
clinical
standard
[
25
],






































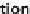








































Search WWH ::

Custom Search