Biology Reference
In-Depth Information
to glycophorin A, Band 3 (anion transporter) and stomatin, each comprising
nearly one million copies per cell, the mean rupture force must correspond
to the uprooting of these proteins.
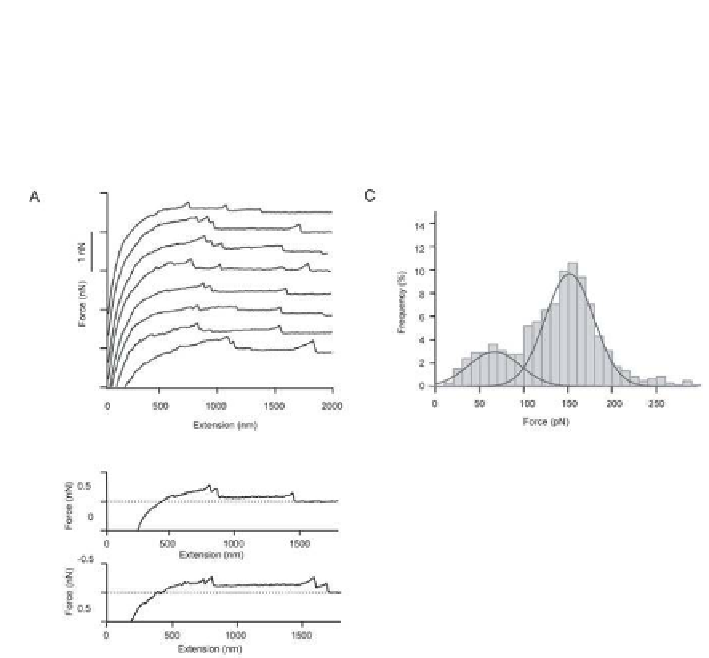
Figure 12.4
shows representative force
curves and the histogram of the inal rupture forces.
54
(a)
(c)
(b)
Figure 12.4.
Force curves obtained on a red blood cell using an AFM probe coated
with covalent cross-linkers. (a) Force-extension curves obtained on native red cell
surface, (b) selected force-extension curves to emphasize the non-linear increase of
the force towards the inal rupture event, (c) histogram of rupture forces with two
peaks with Gaussian itting curves. Reproduced with permission from Afrin
et al.
54
The results of covalent cross-linking experiments indicated that
if the unbinding force of a ligand-receptor pair is in the range of 60-100
pN, the probability of uprooting the receptor protein cannot be neglected.
Although there is no clear picture of how the proteins are extracted from the
membrane, two possible ways can be imagined. In the irst case, proteins are
freed from the lipid bilayer and extracted as lipid free entities, while, in the
second case, the proteins are extracted together with membrane lipids. In
the latter case, breakage of lipid tether trailing behind the ligand-receptor
complex on the AFM probe would take place at any point between the protein
and the cell surface. As a conclusion, it is safe to perform receptor mapping on
a live, unixed cell surface using a ligand that can be unbound with a relatively
weak force of 10-50 pN from its receptor.















Search WWH ::

Custom Search