Biology Reference
In-Depth Information
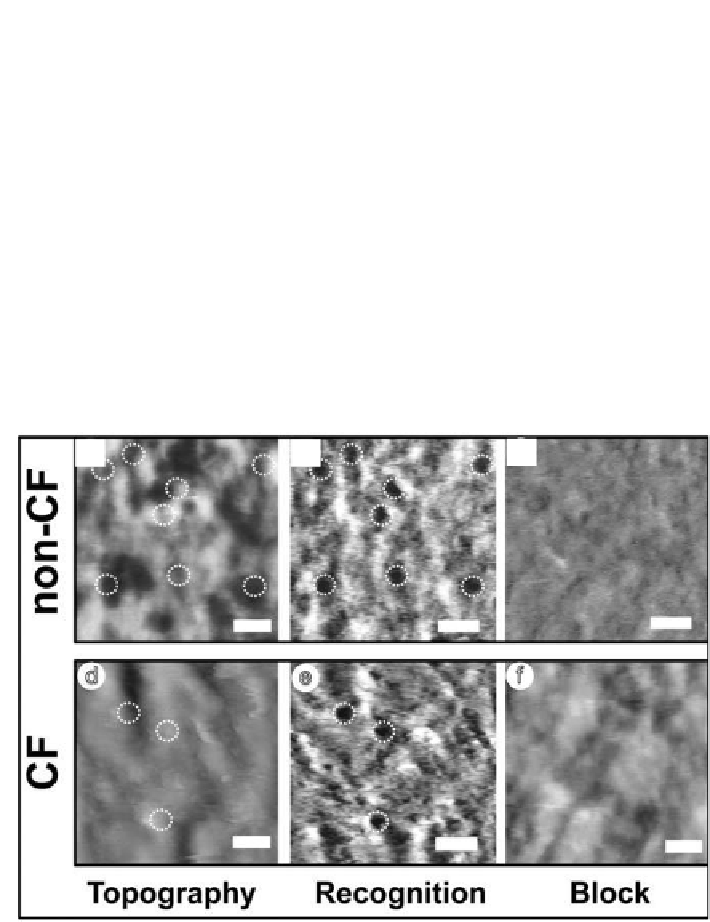
despite the fact that the tip was carrying a tethered antibody. Second, the
simultaneously acquired recognition images
(
Fig. 6.8b,e
)
showed dark spots,
corresponding to interactions between the antibody on the tip and membrane
proteins. These binding sites can be assigned to particular topographical
structures, allowing the identiication of CFTR among the abundance of
different proteins present in the membrane. The most prominent observation
CFTR recognition process was successfully proven in a control experiment
where the antibody-CFTR interaction was blocked. The block resulted in
almost complete abolishment of the recognition spots, conirming clearly
that the recognition events arise from the speciic interaction of anti-CFTR
antibody on the tip with the CFTR on the surface (
Fig. 6.8c,f
)
.
(c)
(a)
(b)
(d)
(e)
(f)
Figure 6.8.
Topography and recognition images of isolated erythrocyte membranes.
TREC imaging topography of a non-CF (a) and of a CF (d) erythrocyte membrane.
Dark spots in the recognition images b and e represent the speciic interaction sites
between the modiied tip (i.e. anti-CFTR antibody tip) and CFTR, corresponding to
the same areas as shown in a and d. The CF membrane (e) clearly reveals fewer
recognition events compared with the non-CF membrane (b). Blocking the membrane
of non-CF (c) and CF (f ) erythrocytes with free anti-CFTR antibody results in the
disappearance of the recognition signals (block eficiency > 90%), conirming the
speciicity of recognition. Scale bar is 200 nm,
z
scale 80 nm.
14














Search WWH ::

Custom Search