Environmental Engineering Reference
In-Depth Information
streams) are in equilibrium with each other. The feed flowrate (
F
) and the mole frac-
tion of our component of interest (
x
0
) are given. There are four unknowns,
L
,
V
,
x
1
,
and
y
1
. Writing two independent mass balances and the equilibrium relation gives three
equations:
Total mass balance:
F
=
L
+
V
(3.34)
Component mass balance:
x
o
F
=
y
1
V
+
x
1
L
(3.35)
Equilibrium relationship:
y
1
=
mx
(assume linear)
.
(3.36)
Therefore, a fourth relationship is needed. Typically, the ratio
L
V
can be set. Now,
expressions for
y
1
and
x
1
can be derived in terms of the mass balances and equilibrium
relationship (see if you can derive these)
/
(1
+
L
/
V
)
x
0
m
(1
+
L
/
V
)
y
1
=
x
0
x
1
=
mV
)
.
(3.37)
(1
+
L
/
mV
)
(1
+
L
/
As stated above, the analysis of a separation process uses mass balances in conjunction
with some specific relation(s) which describe the separation process. For an equilibrium-
stage process, this specific relation is the equilibrium relationship that describes the con-
centration of a component in each phase with respect to each other exiting the stage. Note
that the equations can be solved for a single stage once the equilibrium relationship is
known. It does not have to be a linear one.
Since the separation that is attainable is limited by the equilibrium between the two
outlet streams, the next step would be to put several equilibrium stages in series. This is
shown schematically in Figure 3.29.
The subscripts on
x
and
y
refer to the stage from which they
exit
. The quantities
x
0
and
y
N
+
1
are inlet concentrations to the sequence of equilibrium stages. The flowrates
L
and
V
are assumed constant. This last assumption is useful for developing a basic understanding
but is not a general requirement.
Two mass balances can be written (see Figure 3.29):
y
1
−
V
x
0
L
V
x
N
+
L
y
N
+
1
=
(3.38)
V
,
y
1
V
,
y
N
+ 1
1
2
N
L
,
x
0
L
,
x
N
L
,
x
N
− 1
Figure 3.29
Equilibrium stages in series.

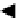










Search WWH ::

Custom Search