Environmental Engineering Reference
In-Depth Information
Results:
Container #
Carbon added (g)
Equilibrium phenol concentration (mg
/
L)
1
0.50
6.00
2
0.64
1.00
3
1.00
0.25
4
2.00
0.08
Solution:
The Langmuir isotherm can be rewritten:
C
e
(
x
1
aK
+
1
a
C
e
,
m
)
=
(3.15)
/
so that a plot (Figure 3.15) of
C
e
/
m
)vs
C
e
is a straight line (remember that
x
is
the amount of phenol adsorbed, which is the equilibrium amount subtracted from the
original amount). A linear regression of the data gives a straight line with a slope of 11
and an intercept of 2.2. Solving for
a
and
K
gives
a
(
x
/
10
−
2
g phenol
=
9
.
1
×
/
g PAC and
K
mg. [Remember the physical meaning of the constant
a
: it will take 91 mg
of adsorbed phenol to completely saturate1gofcarbon.]
Once the constants are determined, the amount of carbon required to obtain an
equilibrium concentration of 0.10 mg
=
4.9 L
/
/
L phenol can be easily determined (be sure not
to mix mg and g units):
mass carbon required = 170 mg for 1 liter solution.
80
60
40
20
0
0
2
4
6
8
e
Figure 3.15
A plot of
C
e
/
(
x
/
m
)vs
C
e
, Example 3.2.
Freundlich isotherm
The Langmuir-Freundlich equation is
(
KC
e
)
1
/
n
=
q
(
KC
e
)
1
/
n
,
(3.16)
1
+





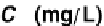










Search WWH ::

Custom Search