Environmental Engineering Reference
In-Depth Information
I
II
III
0
1.0
1.0
0
0
1.0
Relative concentration,
C
/
C
0
IV
V
0
1.0
0
1.0
Relative concentration,
C
/
C
0
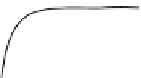
Figure 3.14
The five types of adsorption isotherms.
C
0
is the saturation concen-
tration of solute in fluid phase.
and the adsorption energy distribution for the sites. These derivations can be found in
various references [4-6]. The resulting quality of fit can then provide information about
the adsorption process for the particular sorbate-sorbent pair. The most commonly used
isotherm equations will be presented below.
Langmuir isotherm
Langmuir's theory for deriving an isotherm is a kinetic one, assuming the adsorption
system is in dynamic equilibrium, where the rate of adsorption is equal to that of desorption.
The Langmuir isotherm, described by the following equation, is still the most useful for
data correlation.
x
m
=
aKC
e
q
=
(3.14)
1
+
KC
e
where
x
=
mass of solute adsorbed
m
=
mass of adsorbent
q
=
mass ratio of the solid phase - mass of adsorbed solute per mass of
adsorbent
C
e
=
equilibrium concentration of solute (mass
/
volume)
a
=
mass of adsorbed solute required to completely saturate a unit mass of
adsorbent (constant)
K
=
experimental constant.
Some values of these constants are provided in Table 3.1.








Search WWH ::

Custom Search