Environmental Engineering Reference
In-Depth Information
D
P
F
1
F
2
Membrane
Control
volume for
microscopic
balance
R
B
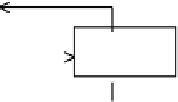
Figure 3.4
Control volume for microscopic balance.
If the actual number of variables specified (values given) is larger than
DF
, the system is
said to be over-specified. Similarly, specifying less variables than
DF
causes the system
to be under-specified. In both situations, a unique solution for the problems could not be
obtained.
Some examples of variables would be composition, temperature, pressure, heat load,
and flowrate of a given phase. If a phase composition is specified as mole fractions, then
the weight fractions would not be a separate set of independent variables since they both
measure composition and are directly related to each other. In other words, given the mole
fractions and the molecular weights of each component (which are constants), the weight
fractions can be calculated.
Some examples of equations are mass balances, energy balances, equilibrium relation-
ships, and equalities (identities) of the temperature and/or pressure of two phases. If the
system contains
C
components, then there are
C
independent mass balances. One example
would be a mass balance for each component. In this case, the total mass balance is not
independent since it is just the sum of the component balances.
Let's do some examples to illustrate this approach.
Figure 3.5 illustrates a batch two-phase system (vapor and liquid) in thermodynamic
equilibrium. As before,
y
i
,
x
i
are the mole fractions of component
i
in the vapor and liquid
phase, respectively. It is assumed that there are
C
components and each is present in both
phases. The system has a constant temperature (
T
) and pressure (
P
).
The number of independent variables (
V
) is:
2
C
component mole fractions (
C
components in each phase and their mole
T
fractions will normally be different in each phase)
P
2
C
+
2










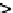









Search WWH ::

Custom Search