Environmental Engineering Reference
In-Depth Information
Purified water
Concentrated salt
water
++
++
+
Anode
-
-
-
-
-
Cathode
++
++
+
-
-
-
-
-
AC A
C
Anion-permeable membrane (A)
Cation-permeable membrane (C)
Saline feed water
Figure 9.12
Two-cell electrodialysis stack.
When a constant voltage is applied to the electrodes, all cations migrate towards the
cathode, and all anions migrate towards the anode. The cations can pass through the cation-
permeable membrane, but they cannot permeate the anion-permeable membrane. The
counter argument applies to anions. Alternate compartments contain ionic concentrations
that are greater or less than the feed solution. These compartments are then combined to
create the brine (waste) stream and the purified water stream.
The membranes are sheets fabricated of a synthetic ion-exchange resin. The cation-
permeable membrane has a fixed negative charge (its fixed exchange sites are anionic).
The cations in solution will enter the membrane when a voltage is applied to the system.
They will not exchange with cations in the membrane because the electrical forces for ion
motion are greater than the attractive forces between the cation and the membrane. Since
the membrane structure is negatively charged, it repels anions. The opposite is true for the
anion-permeable membrane.
The current required for an electrodialysis system can be calculated with Faraday's Law
of electrolysis. One farad (F) (96,500 coulombs) will cause one gram equivalent weight
of a charged species to migrate from one electrode to another:
Equivalents removed
/
unit time
=
QNE
r
,
where
Q
=
solution flowrate
N
=
normality of the solution (equivalents
/
L)
E
r
=
electrolyte removal (fraction of total equivalents).
The current for a single cell is:
FQNE
r
E
c
I
=
,
(9.18)



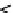






















Search WWH ::

Custom Search