Environmental Engineering Reference
In-Depth Information
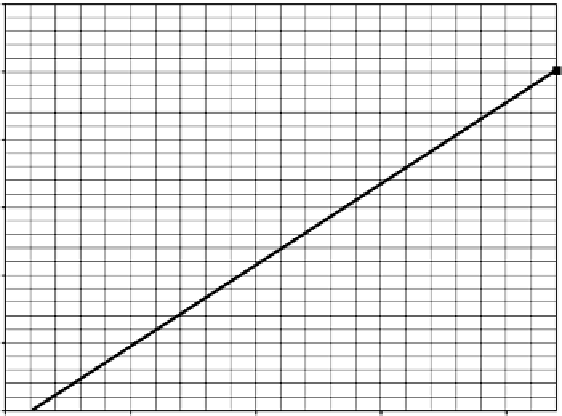
Ion exchange using Langmuir isotherm
60
50
40
30
Equilibrium line
Operating line
20
10
0
0
50
100
150
200
X(mg contaminant
/
L water)
Figure 8.12
Graphical solution, Example 8.2(b).
Ion exchange is a reversible process, and the resin can be regenerated. However, a
resin can be fouled and is susceptible to physical damage, so lifetime is an important
issue.
8.9
Questions
8.1 Describe the difference between a weak and a strong ion-exchange material.
8.2 How could the pH of the fluid to be treated affect the capacity of an ion-exchange
resin?
8.3 What is the primary difference between ion exchange and absorption?
8.4 What is required to completely de-mineralize a water stream through ion exchange?
8.10
Problems
8.1 For the test column and breakthrough curve given in Example 8.1, determine the meq
of Cu
2
+
ion removed per 100 grams of resin on a dry weight basis at the allowable
breakthrough volume,
V
bt
, for
C
a
= 0.05 C
0
. Also, determine the meq of Cu
2
+
ion
removed per 100 grams of resin on a dry weight basis at complete exhaustion. The
dry weight of resin used was 23.24 grams.



Search WWH ::

Custom Search