Environmental Engineering Reference
In-Depth Information
4
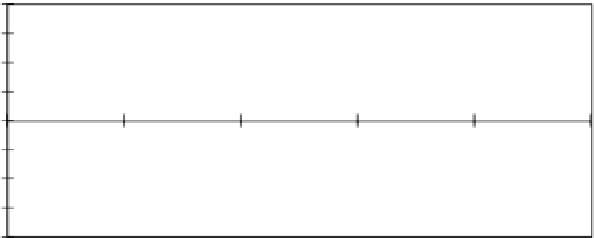
3
y
=
−
0.76x
+
15.3
2
1
0
15
17
19
21
23
25
−
1
−
−
−
V (L)
Figure 8.9
Ion exchange in waste treatment, Example 8.1.
Example 8.2
[15]
Problem:
A certain ion-exchange resin used for treating wastewater contains a finite quantity
of charged groups. Therefore, the equilibrium can be expressed in the same way that
an adsorption equilibrium is described: with an isotherm. Laboratory analysis of this
resin shows that it follows the Langmuir isotherm:
aX
Y
=
X
,
(8.15)
b
+
where
Y
=
amount exchanged (mass contaminant
/
volume resin)
X
=
concentration in solution (mass contaminant
/
volume water)
cm
3
a
=
70 mg
/
b
=
50 mg
/
L.
/
L contaminant,
how much fresh resin is necessary to adsorb 90% of the contaminant? Solve this
part algebraically.
(b) How many countercurrent stages would be required to adsorb 95% of the contam-
inant in the same feed as part (a) using 6.25 cm
3
(a) If you have 1.5 L of an aqueous waste stream containing 220 mg
of pure resin? Solve this part
graphically.
Solution:
(a) A schematic diagram is shown in Figure 8.10.
Contaminant balance: 1.5(220) =
SY
1
+
1
.
5(22)
.
Y
1
.
∴
S
=
297
/
cm
3
Equilibrium:
Y
1
=
70
X
1
/
(50
+
X
1
) where
Y
1
is in mg
/
and
X
1
is in mg
/
L.
cm
3
At
X
1
=
22 mg
/
L
⇒
Y
1
=
21.4 mg
/
resin.















Search WWH ::

Custom Search