Environmental Engineering Reference
In-Depth Information
FLUID PHASE
SORBENT
1
2
PORE
3
4
Figure 7.2
Adsorption mechanisms.
3 Transfer of the adsorbate from the particle surface to the interior of the adsorbent via
the pore network. This step can be accomplished in two ways: pore diffusion (diffusion
through the fluid in the pore); and surface diffusion (the particle travels along the pore
surface).
4 Physical or chemical binding of the adsorbate to the internal surface of the adsorbent.
This step is controlled by the molecular interactions described previously for adsorption.
Steps 1 and 4 are usually the fastest steps, and therefore are not considered to contribute
to the overall rate of adsorption. The rate-determining step is typically Step 3, although
changes in fluid flowrates can affect mass transfer across the fluid-particle boundary layer
(Step 2).
For fixed beds, Step 2 can be described by:
17
Re
−
0
.
415
j
=
1
.
10
<
Re
<
2500
(7.1)
k
v
s
Sc
0
.
067
=
=
Chilton-Colburn
j
factor
(7.2)
ρ
f
ν
s
d
p
µ
where
Re
=
=
Reynolds number
µ
Sc
=
D
AB
=
Schmidt number
ρ
ρ
f
=
fluid density
µ
=
fluid viscosity
v
s
=
fluid superficial velocity
d
p
=
particle diameter
k
=
mass transfer coefficient
D
AB
=
diffusion coefficient of sorbate in fluid.
High values of
k
correspond to less mass transfer resistance for this step. One way to in-
crease
k
is to increase
v
s
. This can cause problems, though, since the contact time in the bed
would be reduced. This can lead to breakthrough with a larger portion of the bed
unused.







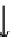



Search WWH ::

Custom Search