Environmental Engineering Reference
In-Depth Information
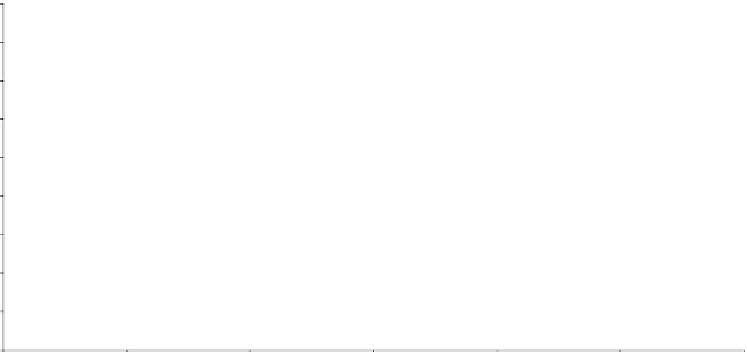
Equilibrium line
0.18
0.16
0.14
(
X
0
,
Y
1
)
Operating line
0.12
0.1
1
0.08
2
0.06
0.04
3
4
0.02
5
0
0
0.02
0.04
0.06
0.08
0.1
0.12
(
X
N
,
Y
N
+1
)
X
(Mass ratio of solvent in liquid)
Figure 6.9
X
-
Y
plot for stripping column, Example 6.3.
The term
L
/
mV
=
A
is called the absorption factor. It is often written as
L
/
KV
where
y
=
Kx
.Itwould be useful to determine the ratio
y
1
y
N
+
1
=
fraction of solute in entering gas
not
adsorbed
.
The above equation can be rearranged to obtain:
y
1
y
N
+
1
=
A
−
1
1
.
(6.8)
A
N
+
1
−
For absorption, a large value of
A
is better. Since
m
(or
K
)isinthe denominator, a small
value of
m
(or
K
) means a small mole fraction in the gas phase (
y
) relative to the value of
x
. Solute favors the liquid phase.
For stripping, an analogous equation can be derived:
x
1
x
N
+
1
=
S
−
1
1
=
fraction of entering solute in liquid
not
stripped,
(6.9)
S
N
+
1
−
1
A
.
In this case, a large value of
S
is better. This translates to a large value of
m
(or
K
). Can
you see why?
mV
L
KV
L
where
S
=
=
=
Example 6.4
Problem:
Referring to Example 6.3 for a stripping column,
L
=
500 lb
/
hr
,
V
=
500 lb
/
hr
,
m
=
5 and
x
N
+
1
=
1
This value of
x
N
+
1
is somewhat higher than what is considered
dilute but it is still reasonable to use the analytical approach as a first estimate. For
N
.
0
.
1
.
=
5, what is
x
1
?

















Search WWH ::

Custom Search