Environmental Engineering Reference
In-Depth Information
G
,
Y
1
L
,
X
0
1
N
=?
G
,
Y
N
+1
L
,
X
N
=?
Figure 6.4
Schematic for absorption column, Example 6.2.
Y
N
+1
0.5
Y
Slope = 0.68
Y
1
=
0.005
0.36
0.73
X
Figure 6.5
Graphical determination of equilibrium stages, Example 6.2.
solvent enters with
X
a
solute concentration. The maximum exiting liquid concentration
and the corresponding minimum liquid flow rate are given by line
ab
.
Because
ab
intersects the equilibrium line, no further separation can occur. In other
words, the equilibrium concentration of the solute in the solvent cannot be exceeded. This
is the minimum liquid flowrate. Just as with the minimum reflux ratio, this situation is
hypothetical because an infinite number of equilibrium stages (or an infinitely tall column)
would be required.
Despite its theoretical nature, it is still useful to know the minimum liquid flowrate
because it corresponds to the maximum concentration of the solute in the mass separating
agent (largest value of
X
). This becomes important when the solvent MSA is recovered
downstream for recycle back into the system. When the solute is highly concentrated
in the MSA, there is less total material that must be processed in the solvent recovery
system. When the solute is dilute in the MSA, more material must be processed in the
solvent recovery system. However, fewer theoretical stages are required in the absorption
column. A good design will balance these two factors. Also, a typical design will specify
the actual
L
/
G
as a multiple of the minimum value.

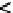

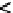
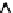
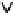














Search WWH ::

Custom Search