Environmental Engineering Reference
In-Depth Information
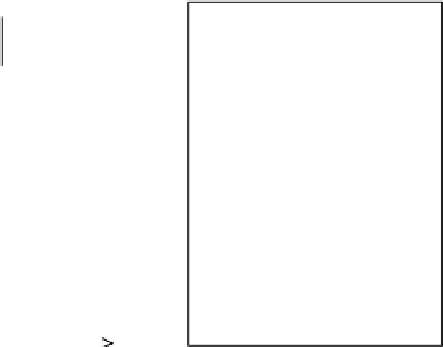
G
= gas stream
L
= liquid stream
Y
1
= solute intreated gas
stream (mole ratio)
X
0
= solute in inlet liquid
stream (mole ratio)
Y
N
+1
= solute in inlet gas
stream (mole ratio)
X
N
= solute in exit liquid
stream (mole ratio)
Subscripts correspond to
stage number of
exit
stream
G, Y
1
L, X
0
1
j
Y
j+1
X
j
N
G
,
Y
N
+1
L
,
X
N
Figure 6.2
Absorption column schematic.
We cannot, however, say that overall flowrates of gas and liquids are constant (except for
very dilute solutions) because a significant amount of solute may be absorbed. This would
increase the total flowrate of liquid, while reducing that of the gas. The compositions of
the solute must therefore be expressed in mole ratios so that the basis (denominator) is
constant. Mole ratios are related to mole fractions by the equations:
y
moles solute
moles insoluble carrier gas
=
Y
y
=
1
−
and
x
moles solute
moles pure liquid
.
X
=
x
=
1
−
So that the solute balance is:
Y
j
+
1
G
+
X
0
L
=
X
j
L
+
Y
1
G
.
(6.5)
Solving for
Y
j
+
1
gives:
Y
1
−
G
X
0
L
G
X
j
+
L
Y
j
+
1
=
,
(6.6)
the operating line for absorption. As in distillation analysis, an operating line can be
plotted if
L
,
G
, and a single point are known. The operating line is a straight line with
slope
L
G
on a
Y
vs
X
diagram. The equilibrium line can be curved. For absorption, the
Y
intercept is greater than zero so the operating line is
above
the equilibrium line for this type
of plot.
Note that two additional assumptions are necessary to neglect the energy balances
for absorption processes. They are that the heat of absorption is negligible and that the
operation is isothermal.
/














Search WWH ::

Custom Search