Environmental Engineering Reference
In-Depth Information
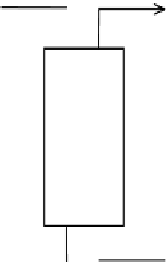
Feed (diluent)
F
D
,x
D
Extract (spent solvent)
E, y
1
1
N
Raffinate (product)
R, x
N
Solvent
F
S
,y
N
+1
Figure 5.24
Schematic for water purification, Example 5.5.
diluent phase, while y's are used to describe the amount of the same solute in the solvent
phase.
Mass balance around control volume:
Y
1
−
X
0
F
D
F
S
F
D
F
S
F
S
Y
1
+
F
D
X
j
=
F
S
Y
j
+
1
+
F
D
X
0
⇒
Y
j
+
1
=
X
j
+
.
(5.18)
Since
F
D
and
F
S
are constant for an immiscible system, the operating line is a straight
line.
Equilibrium data:
Equilibrium data for dilute extractions are usually given in terms of the distribution
ratio,
Y
A
X
A
K
d
=
(5.19)
in weight or mole fractions.
K
d
is usually constant for very dilute systems, but can
become a function of concentration. It is also temperature and pH dependent.
Example 5.5: countercurrent immiscible extraction
Problem:
Figure 5.24 is a schematic of a water purification system. A 20 wt% mixture of acetic
acid in water is to be extracted with 1-butanol. The outlet concentrations of acetic acid
should be 5 wt% in the water phase and 10 wt% in the 1-butanol phase. Pure solvent
is used. Find the number,
N
,ofequilibrium stages required and the ratio,
F
D
/
F
S
,of
water to 1-butanol. The distribution coefficient,
K
d
, for this system is 1.6.
Solution:
Given:
K
d
=
1
.
6
x
D
=
0
.
20
y
1
=
0
.
10








Search WWH ::

Custom Search