Environmental Engineering Reference
In-Depth Information
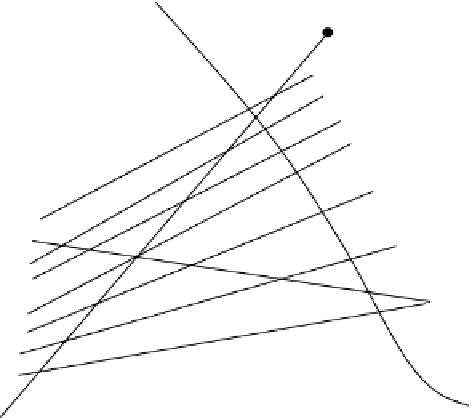
4 Find the line thr
ough
O
1
M
to get
V
N
(it's on the saturation curve).
5 Extend the lines
V
0
O
1
and
V
N
O
N
+
1
: the intersection is the
point.
6 Stepping off stages:
-
Use a tie-line fro
m
O
1
to find
V
1
.
-
Draw and extend
V
1
.Itcrosses the solubility curve at
O
2
.
-
Use the tie-line from
O
2
to find
V
2
, etc.
50
45
40
O
N
+1
O
7
O
6
O
5
O
4
35
30
25
V
7
O
3
20
V
6
O
2
V
5
N
M
15
V
4
O
1
10
V
3
V
2
5
V
1
0
0
20
40
60
80
100
∆
wt % water (component
A
)
V
0
Conjugate line
Figure
5.14
Countercurrent
cascade:
water-acetic
acid-isopropyl
ether,
Example 5.2.
5.7
Minimum solvent flowrate
Remember that:
V
0
O
N
+
1
=
solvent flowrate
feed flowrate
.
(5.12)
Note that in Figure 5.13, this ratio decreases as the point
M
moves toward the point
V
0
(lever-arm
rule). This causes a decrease in the solute concentration of
V
N
. When the line
V
0
O
N
+
1
changes such that it falls exactly on a tie-line (equilibrium and operating lines























































Search WWH ::

Custom Search