Environmental Engineering Reference
In-Depth Information
K
'
(a)
(b)
L
'
J
'
Reboil
Reboil
L
y
K
J
x
B
x
F
x
D
x
B
x
F
x
D
x
x
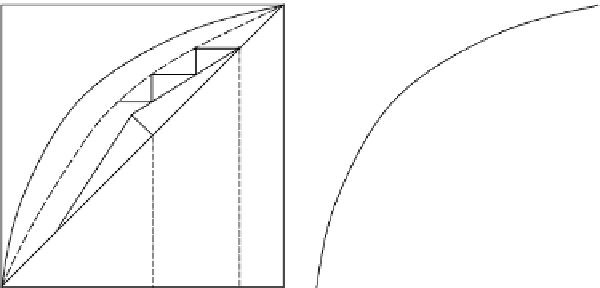
Figure 4.21
Murphree efficiencies: (a) vapor phase, E
MV
, (b) liquid phase, E
ML
.
where
y
n
=
actual composition of
V
phase leaving the stage
y
n
=
composition of hypothetical
V
phase that would be in equilibrium with
L
phase leaving actual stage.
This method is more meaningful than the overall efficiency because it is based on the
difference between true operating and hypothetical equilibrium vapor-phase concentra-
tions. However, Murphree efficiencies also vary from stage to stage and require difficult
measurement of process variables.
The Murphree ef
ficiency i
s the ratio of distance between the operating line and the
equilibrium line (
=
[
JK
/
JL
×
100]% in Figure 4.21(a)). Similarly, the Murphree liquid
efficiency is given by:
x
n
−
x
n
+
1
E
ML
=
x
n
+
1
×
100(%)
,
x
n
−
[J
K
/
J
L
×
100]% in Figure 4.21(b)).
Note:
Remember that a reboiler is an equilibrium stage, even though the other stages in a
column will not reach equilibrium if they are not 100% efficient. Therefore, the last stage
(the one which gives
x
B
) will appear on the graph at the solid equilibrium curve, not on
the dashed curve. The dashed curve is a “pseudo-equilibrium” which describes the two
phases when they don't have a chance to completely reach equilibrium.
(
=
Example 4.6: plutonium stabilization at Los Alamos National Laboratories
[4]
Problem:
Currently, the nitric acid used in plutonium stabilization operations at a particular
facility is evaporated to remove dissolved solids, assayed for radioactive content and
then sent via underground pipe to a low-level waste handling facility. The acid stream
is then neutralized with caustic to remove radioactivity and the resulting solids are
immobilized in cement as a TRU (trans-uranic waste). The filtrate is then sent for

















Search WWH ::

Custom Search