Geology Reference
In-Depth Information
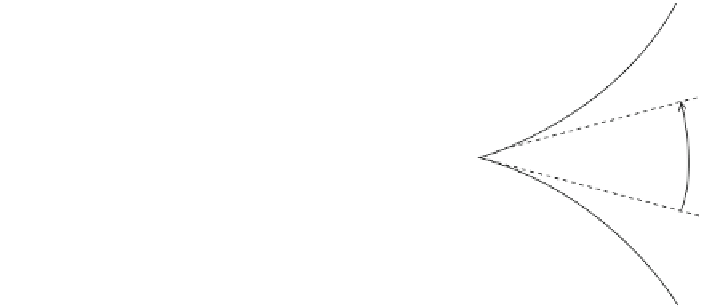
Fig. 1.1 Defining the
dihedral angle h between the
solid-liquid (S-L) interfaces
and their junction with a
solid-solid interface (S-S)
s
L
s
of the grains occurs will become larger than 21 % when h exceeds zero. The
case 0\h\60
might be expected to be common where the melt is moderately
similar chemically to the solid and c
SL
is somewhat less than c
SS
because of
greater ease of atomic configurational adjustment and the liquid-solid boundary
being the predominating factor. The observations of Waff and Bulau (
1979
,
1992
) on annealed olivine-basalt mixtures fall in this category, the values of c
SS
and c
SL
being about 0.9 and 0.5 J m
-2
, respectively (Cooper and Kohlstedt
1982
), and the grain boundaries themselves being shown to be free of melt
within a resolution of 2 lm (Vaughan and Kohlstedt
1982
).
3. When c
SL
[ c
SS
, then h [ 60
and the melt will tend to be segregated in tet-
rahedral pockets at 4-grain junctions (grain corners). The volume fraction for
interconnection of the melt at equilibrium increases as h increases, up to about
30 % for h
¼
180
, and substantially larger amounts of melt are needed to
surround the grains completely for all values of h in this range. The case
h [ 60
is
likely
to
arise
when
melt
and
solid
are
strongly
dissimilar
chemically.
In practice, long annealing times may be necessary to achieve the equilibrium
configurations (*200 h at 1,240 C for grain size *50 lm in Waff and Bulau's
study). Many specimens in laboratory studies may therefore have non-equilibrium
distributions of melt in the form of widespread grain boundary films and irregu-
larly shaped pockets of melt occurring wherever the melt was formed initially.
Similar considerations apply to interfaces between solid phases, although tor-
que terms may sometimes have to be included in the dependence of interfacial
energy on interface orientation (Cahn
1982
). Here, however, equilibrium may be
approached even more slowly than for the liquid-solid case and there may be more
situations where anisotropy cannot be neglected.
The nature of void or pore structure associated with grain boundaries is of
paramount importance in determining the permeability of a rock but it can also
influence the mechanical properties; for example, at low temperatures, voids may
act as nuclei for microcracks and at high temperatures they may contain fluids that
promote diffusional creep. Void space may be of many origins and have many