Biomedical Engineering Reference
In-Depth Information
Besides size, the surface charge of nanocarriers also influences their
penetration into tumor tissues. Cationic liposomes (150 nm) were observed not
able to travel far into the tumor interstitium.
169
Recently, Forbes et al.compared
the penetration of oppositely charged gold nanoparticles (+30 vs. 236 mV, 6 nm)
into cylindroidal cell aggregates.
170
Cationic nanoparticles were taken up by the
proliferating cells on the periphery of the cylindroids, whereas anionic
nanoparticles were better at penetrating the extracellular matrix and entered
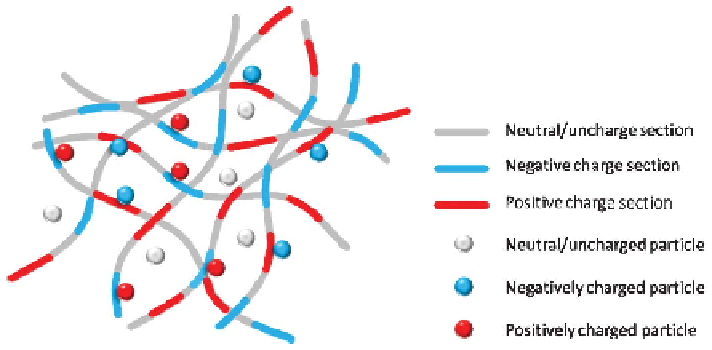
hypoxic necrotic cells in the core of the mass. As a matter of fact, the extracellular
matrix presents as an effective electrostatic bandpass, suppressing the diffusive
motion of both positively and negatively charged objects, which allows
uncharged particles to easily diffuse through while effectively trapping charged
particles (Figure 3.10).
171
Jain et al. demonstrated that the optimal particles for
delivery to tumors should be neutral after exiting the blood vessels.
172
Another issue that needs addressing is affinity.
173
The affinity plays an
important role in antibody-based tumor targeting nanocarriers. It was visualized
that the antibody distributed mostly in perivascular regions rather than homo-
geneously in tumor cells.
174
Reports revealed there was an inverse relationship
between affinity and penetration, i.e. the antigen-antibody interaction in the
tumor tissue imposed a binding-site barrier that retarded antibody penetration
and caused a heterogeneous distribution.
175-177
The higher the affinity of binding
and the higher antigen density caused fewer free molecules to be able to penetrate
farther into the tumor interstitium.
175,176
Increasing the antibody dose gave better
penetration and more uniform distribution.
175
Therefore, it is clear that to deliver a sufficient drug concentration to the
tumor center region lacking vascular perfusion, where the most aggressive and
resistant cells reside, the nanocarrier should not release the carried drug after
d
n
4
y
3
n
g
|
2
Figure 3.10
Scheme of the ECM exerts the filtering function in tumor tissue.
Charged particles (red, blue) are trapped in the respective region of
opposite charge (blue, red), while neutral particles (gray) can diffuse
nearly unhindered.