Biomedical Engineering Reference
In-Depth Information
d
n
4
y
3
n
g
|
2
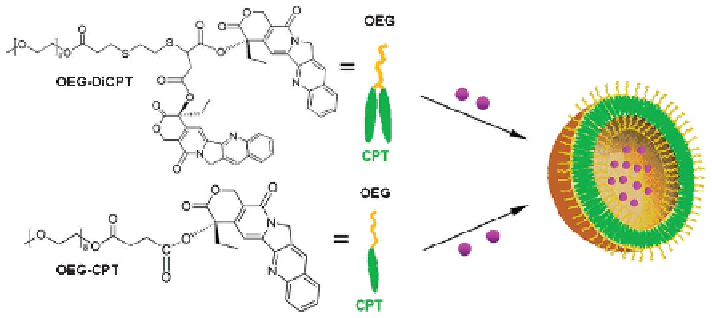
Figure 3.5
Amphiphilic CPT prodrugs (OEG-CPT and OEG-DiCPT) and their self-
assembly into nanocapsules. Reprinted with permission from ref. 28.
Copyright 2010 American Chemical Society.
The main disadvantage of such conjugation approaches is that they may
change the drug chemical structure,
30
which in turn may reduce the
pharmaceutical efficacy, not to mention the need for extensive preclinical
tests and clinical trials before acquiring FDA approval.
(3) Core- or shell-crosslinked micelles
The third approach aimed at reducing the burst release is crosslinking the
core or the corona shell of micelles. For example, Wooley et al. developed
methods for fabricating shell-crosslinked micelles.
31
In order to de-crosslink
the shell to allow drug release at the target site, a linker labile in the presence of
intracellular glutathione (GSH) was used.
32
As intended, such crosslinked
shells inhibited drug diffusion from the micelles, and hence reduced burst
release. However, such a crosslinked shell becomes more rigid and hence loses
its ability to repel serum proteins or other biomacromolecules,
33
and thus may
not continue to be stealthy in circulation.
Covalent crosslinking of the micelle hydrophobic core can therefore be a
preferable approach.
34,35
For instance, crosslinked micelles consisting of PEG-
b-poly(acryloyl carbonate)-b-poly(
D
,
L
-lactide) (PEG-PAC-PDLLA) had high
stability and significantly inhibited PTX release at low micelle concentrations
compared to the non-crosslinked controls.
36
Lavasanifar et al. applied click
chemistry and developed hydrolysable core-crosslinked PEG-b-poly(a-propar-
gyl carboxylate-e-caprolactone) (PEG-PPCL) micelles that exhibited a lower
degree of PTX burst release than equivalent non-crosslinked micelles.
37
When
the crosslinked core had disulfide linkers, it was shown to hold the drug tightly
but release it quickly once in the tumor cell, due to the cleavage of the
crosslinkages by intracellular GSH.
38
Similarly, thiolated Pluronic copolymer
(Plu-SH) was demonstrated to form core-crosslinked micelles that were
reversible via dithiothreitol (DTT)-breakable disulfide bonds, which inhibited
the premature release in an aqueous solution.
39